order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
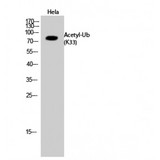
Ubiquitin
Ubiquitin is a small (8.5 kDa) regulatory protein that has been found in almost all tissues (ubiquitously) of eukaryotic organisms. It was discovered in 1975 by Goldstein and further characterized throughout the 1970s and 1980s. There are four genes in the human genome that produce ubiquitin: UBB, UBC, UBA52 and RPS27A. Ubiquitination is a post-translational modification (an addition to a protein after it has been made) where ubiquitin is attached to a substrate protein. The addition of ubiquitin can affect proteins in many ways: It can signal for their degradation via the proteasome, alter their cellular location, affect their activity, and promote or prevent protein interactions. Ubiquitination is carried out in three main steps: activation, conjugation, and ligation, performed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s), respectively. The result of this sequential cascade binds ubiquitin to lysine residues on the protein substrate via an isopeptide bond or to the amino group of the protein's N-terminus via a peptide bond. The protein modifications can be either a single ubiquitin protein (monoubiquitination) or a chain of ubiquitin (polyubiquitination). The ubiquitination bonds are always formed with one of the seven lysine residues from the ubiquitin molecule. These 'linking' lysines are represented by a "K" (which is the one-letter amino acid notation of lysine) and a number, referring to its position in the ubiquitin molecule. First, a ubiquitin molecule is bonded by its C-terminus to a specific lysine residue (e.g. K48, K29, K63,…) on the target protein. Poly-ubiquitination occurs when the C-terminus of another ubiquitin, will be linked again to a lysine residue (for example again K48 or K29) on the previously added ubiquitin molecule, forming a chain. This process repeats several times, leading to the addition of several ubiquitins. Only poly-ubiquitination on defined lysines, mostly on K48 and K29, is related to degradation with the proteasome (referred to as the "molecular kiss of death"), while other polyubiquitinations (e.g. on K63, K11, K6) and monoubiquitinations may regulate processes such as endocytic trafficking, inflammation, translation and DNA repair.
Ubiquitin Target Details
| Target Name: | Ubiquitin |
Publications (9)
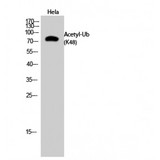
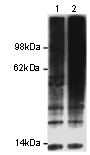
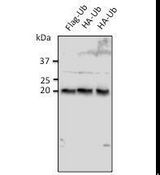
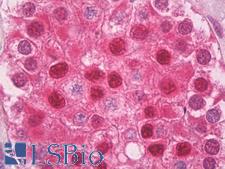
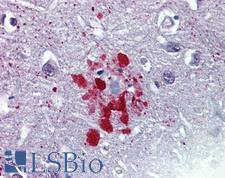
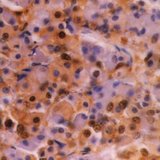
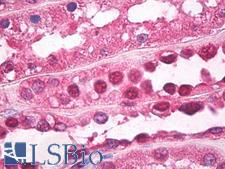
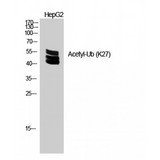


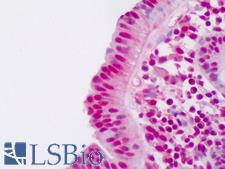
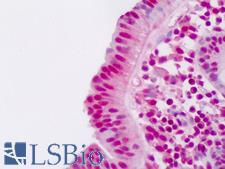
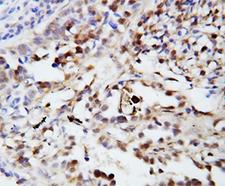
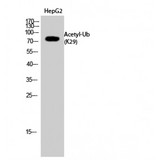

If you do not find the reagent or information you require, please contact Customer.Support@LSBio.com to inquire about additional products in development.










