order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
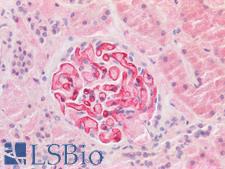
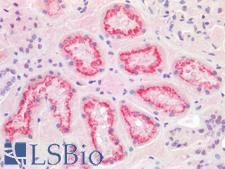
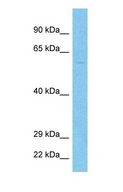
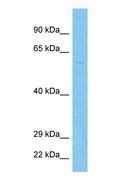
TMPRSS2 / Epitheliasin
transmembrane protease, serine 2
Serine protease that proteolytically cleaves and activates the viral spike glycoproteins which facilitate virus-cell membrane fusions; spike proteins are synthesized and maintained in precursor intermediate folding states and proteolysis permits the refolding and energy release required to create stable virus-cell linkages and membrane coalescence. Facilitates human SARS coronavirus (SARS-CoV) infection via two independent mechanisms, proteolytic cleavage of ACE2, which might promote viral uptake, and cleavage of coronavirus spike glycoprotein which activates the glycoprotein for cathepsin L-independent host cell entry. Proteolytically cleaves and activates the spike glycoproteins of human coronavirus 229E (HCoV-229E) , human coronavirus EMC (HCoV-EMC), SARS-CoV and SARS-CoV-2, and the fusion glycoproteins F0 of Sendai virus (SeV), human metapneumovirus (HMPV), human parainfluenza 1, 2, 3, 4a and 4b viruses (HPIV). Essential for spread and pathogenesis of influenza A virus (strains H1N1, H3N2 and H7N9); involved in proteolytic cleavage and activation of hemagglutinin (HA) protein which is essential for viral infectivity.
| Gene Name: | transmembrane protease, serine 2 |
| Family/Subfamily: | Protease , Serine S1 |
| Synonyms: | TMPRSS2, Epitheliasin, PRSS10, Serine protease 10, PP9284, host protease for SARS-CoV-2 replication |
| Target Sequences: | NM_005656 NP_005647.3 O15393 |
Publications (4)
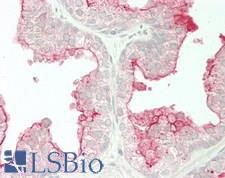









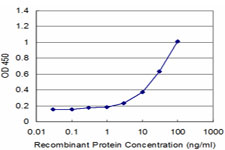
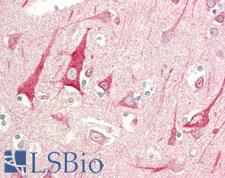


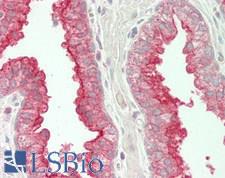

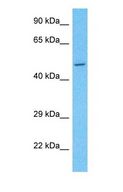
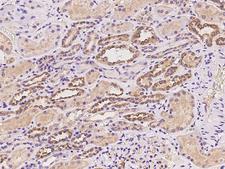
If you do not find the reagent or information you require, please contact Customer.Support@LSBio.com to inquire about additional products in development.











