order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
RFWD2 / COP1
ring finger and WD repeat domain 2, E3 ubiquitin protein ligase
E3 ubiquitin-protein ligase that mediates ubiquitination and subsequent proteasomal degradation of target proteins. E3 ubiquitin ligases accept ubiquitin from an E2 ubiquitin-conjugating enzyme in the form of a thioester and then directly transfers the ubiquitin to targeted substrates. Involved in JUN ubiquitination and degradation. Directly involved in p53 (TP53) ubiquitination and degradation, thereby abolishing p53-dependent transcription and apoptosis. Ubiquitinates p53 independently of MDM2 or RCHY1. Probably mediates E3 ubiquitin ligase activity by functioning as the essential RING domain subunit of larger E3 complexes. In contrast, it does not constitute the catalytic RING subunit in the DCX DET1-COP1 complex that negatively regulates JUN, the ubiquitin ligase activity being mediated by RBX1. Involved in 14-3-3 protein sigma/SFN ubiquitination and proteasomal degradation, leading to AKT activation and promotion of cell survival. Ubiquitinates MTA1 leading to its proteasomal degradation.
| Gene Name: | ring finger and WD repeat domain 2, E3 ubiquitin protein ligase |
| Synonyms: | RFWD2, COP1, HCOP1, Putative ubiquitin ligase COP1, RP11-318C24.3, RNF200, RING finger protein 200 |
| Target Sequences: | NM_022457 NP_071902.2 Q8NHY2 |
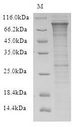
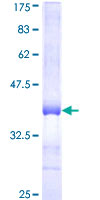
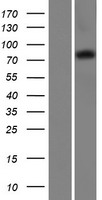
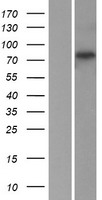
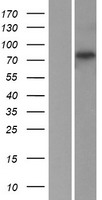
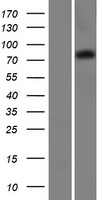
If you do not find the reagent or information you require, please contact Customer.Support@LSBio.com to inquire about additional products in development.









