order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
HSP40
DNAJ / Hsp40 / Heat Shock Protein 40
Chaperone DnaJ, also known as Hsp40 (heat shock protein 40 kD), is a molecular chaperone protein. It is expressed in a wide variety of organisms from bacteria to humans. Molecular chaperones are a diverse family of proteins that function to protect proteins from irreversible aggregation during synthesis and in times of cellular stress. The bacterial molecular chaperone DnaK is an enzyme that couples cycles of ATP binding, hydrolysis, and ADP release by an N-terminal ATP-hydrolizing domain to cycles of sequestration and release of unfolded proteins by a C-terminal substrate binding domain. Dimeric GrpE is the co-chaperone for DnaK, and acts as a nucleotide exchange factor, stimulating the rate of ADP release 5000-fold. DnaK is itself a weak ATPase; ATP hydrolysis by DnaK is stimulated by its interaction with another co-chaperone, DnaJ. Thus the co-chaperones DnaJ and GrpE are capable of tightly regulating the nucleotide-bound and substrate-bound state of DnaK in ways that are necessary for the normal housekeeping functions and stress-related functions of the DnaK molecular chaperone cycle. This family of proteins contain a 70 amino acid consensus sequence known as the J domain. The J domain of DnaJ interacts with Hsp70 heat shock proteins. DnaJ heat-shock proteins play a role in regulating the ATPase activity of Hsp70 heat-shock proteins.
HSP40 Target Details
| Target Name: | DNAJ / Hsp40 / Heat Shock Protein 40 |
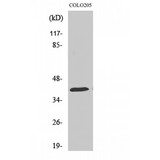
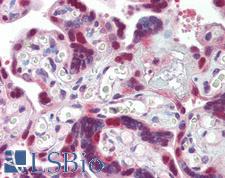
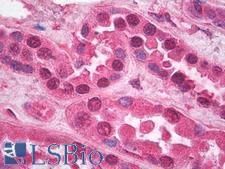

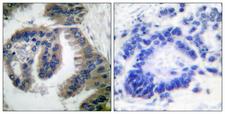
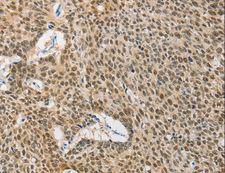









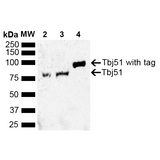
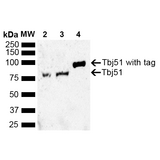
If you do not find the reagent or information you require, please contact Customer.Support@LSBio.com to inquire about additional products in development.










