order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
CRYBA2
Human crystallin, beta A2 (CRYBA2), transcript variant 2, mRNA.
Crystallins are separated into two classes: taxon-specific, or enzyme, and ubiquitous. The latter class constitutes the major proteins of the vertebrate eye, which function to maintain the transparency and refractive index of the lens. Since lens central fiber cells lose their nuclei during development, these crystallins are made and then retained throughout life, making them extremely stable proteins. Mammalian lens crystallins are divided into alpha, beta, and gamma families; beta and gamma crystallins are also defined as a superfamily. Alpha and beta families are further divided into acidic and basic groups. Seven protein regions exist in crystallins: four homologous motifs, a connecting peptide, and N- and C-terminal extensions. Beta-crystallins, the most heterogeneous, differ by the presence of the C-terminal extension (present in the basic group but absent in the acidic group). Beta-crystallins form aggregates of different sizes and are able to form homodimers through self-association or heterodimers with other beta-crystallins. This gene is a beta acidic group member. Three alternatively spliced transcript variants encoding identical proteins have been reported.
| Gene Name: | Human crystallin, beta A2 (CRYBA2), transcript variant 2, mRNA. |
| Synonyms: | CRYBA2 |
| Target Sequences: | NM_057093 NP_476434.1 P53672 |
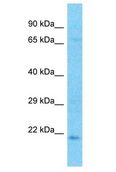
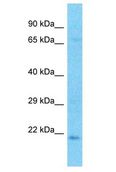
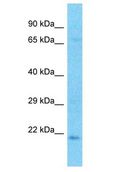
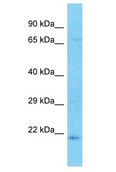
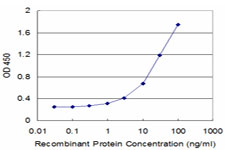
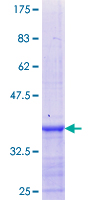
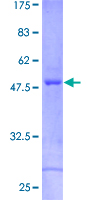



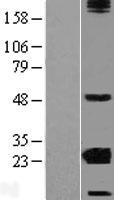
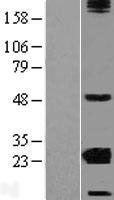
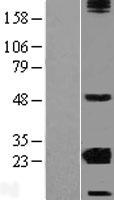
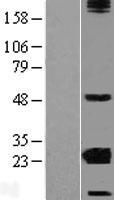
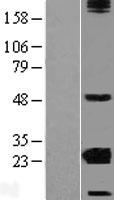
If you do not find the reagent or information you require, please contact Customer.Support@LSBio.com to inquire about additional products in development.









