order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Alpha SNAP
N-ethylmaleimide-sensitive factor attachment protein, alpha
The 'SNARE hypothesis' is a model explaining the process of docking and fusion of vesicles to their target membranes. According to this model, membrane proteins from the vesicle (v-SNAREs) and proteins from the target membrane (t-SNAREs) govern the specificity of vesicle targeting and docking through mutual recognition. Once the 2 classes of SNAREs bind to each other, they form a complex that recruits the general elements of the fusion apparatus, namely NSF (N-ethylmaleimide-sensitive factor) and SNAPs (soluble NSF-attachment proteins), to the site of membrane fusion, thereby forming the 20S fusion complex. Alpha- and gamma-SNAP are found in a wide range of tissues and act synergistically in intra-Golgi transport. The sequence of the predicted 295-amino acid human protein encoded by NAPA shares 37%, 60%, and 67% identity with the sequences of yeast, Drosophila, and squid alpha-SNAP, respectively. Platelets contain some of the same proteins, including NSF, p115/TAP, alpha-SNAP, gamma-SNAP, and the t-SNAREs syntaxin-2 and syntaxin-4, that are used in many vesicular transport processes in other cell types. Platelet exocytosis uses a molecular mechanism similar to that used by other secretory cells, such as neurons, although the proteins used by the platelet and their modes of regulation may be quite different.
| Gene Name: | N-ethylmaleimide-sensitive factor attachment protein, alpha |
| Synonyms: | NAPA, Alpha SNAP, Alpha-SNAP, SNAP-alpha, SNAPA |
| Target Sequences: | NM_003827 NP_003818.2 P54920 |
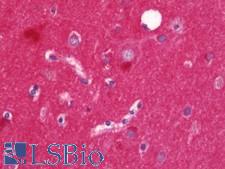
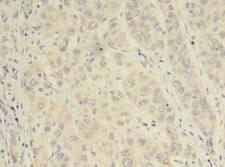
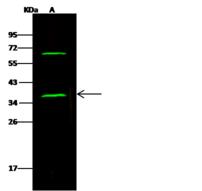
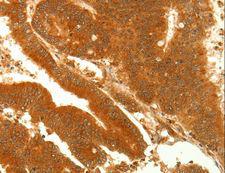
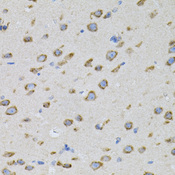
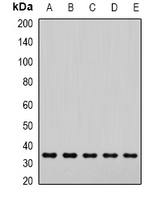








If you do not find the reagent or information you require, please contact Customer.Support@LSBio.com to inquire about additional products in development.











