Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
Products
Antibodies
ELISA and Assay Kits
Research Areas
Infectious Disease
Resources
Purchasing
Reference Material
Contact Us
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
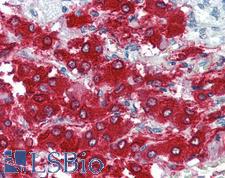
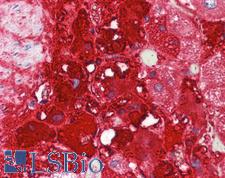
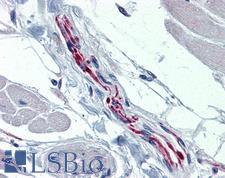
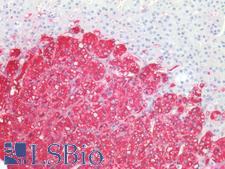
PathPlusTM TH / Tyrosine Hydroxylase Antibodies
TH (tyrosine hydroxylase) is an enzyme that converts L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA), a dopamine precursor. It is the rate-limiting enzyme in the synthesis of catecholamines and plays a key role in the physiology of adrenergic neurons. TH is encoded by four distinct mRNAs produced by alternative splicing of a single primary transcript, and expression of the mRNAs varies in different parts of the nervous system. Mutations in TH are the cause of autosomal recessive Segawa syndrome and are thought to be involved in Parkinson’s disease and schizophrenia. TH deficiency leads to dopa-nonresponsive dystonia and progressive encephalopathy via an impaired ability to synthesize epinephrine, norepinephrine and dopamine. In immunohistochemistry of normal tissue, TH has cytoplasmic positivity in the adrenal medulla, in peripheral sympathetic nerve fibers, and in the caudate nucleus in the brain.
References: Nature Structural Biology. 1997. 4 (7): 578–85, PMID 9228951; CNS Neurol Disord Drug Targets. 2012 Jun 1;11(4):395-409, PMID: 22483313; Arch Biochem Biophys. 2011 Apr 1;508(1):1-12, PMID: 21176768; Ann Neurol. 2003;54 Suppl 6:S56-65, PMID: 12891655
4 PathPlusTM Antibodies
☰ Filters
Products
Antibodies
(4)
Type
Primary
(4)
Target
TH / Tyrosine Hydroxylase
(4)
Reactivity
Human
(4)
Mouse
(2)
Rat
(3)
Cat
(1)
Ferret
(1)
Application
IHC
(3)
IHC-Fr
(3)
IHC-P
(4)
WB
(4)
ELISA
(1)
IF
(4)
IP
(1)
Host
rabbit
(3)
mouse
(1)
Product Group
PathPlus Neuro
(4)
Isotype
IgG
(1)
IgG2a
(1)
Clonality
monoclonal mc
(1)
polyclonal pc
(3)
Clone
OTI3H3
(1)
Format
Unconjugated
(4)
Epitope
pSer40
(1)
Publications
No
(4)

Neuroscience
TH / Tyrosine Hydroxylase Rabbit anti-Rat Polyclonal (pSer40) Antibody
Rat, Human
IF, IHC, IHC-Fr, IHC-P, WB
Unconjugated
50 µl/$460

Neuroscience
TH / Tyrosine Hydroxylase Rabbit anti-Rat Polyclonal Antibody
Mouse, Rat, Cat, Ferret, Human
ELISA, IF, IHC, IHC-Fr, IHC-P, IP, WB
Unconjugated
50 µl/$485

Neuroscience
TH / Tyrosine Hydroxylase Rabbit anti-Rat Polyclonal Antibody
Rat, Human
IF, IHC, IHC-Fr, IHC-P, WB
Unconjugated
50 µl/$375

Neuroscience
TH / Tyrosine Hydroxylase Mouse anti-Human Monoclonal (OTI3H3) Antibody
Mouse, Human
IF, IHC-P, WB
Unconjugated
50 µl/$375
Viewing 1-4
of 4
product results











