Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
Products
Antibodies
ELISA and Assay Kits
Research Areas
Infectious Disease
Resources
Purchasing
Reference Material
Contact Us
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
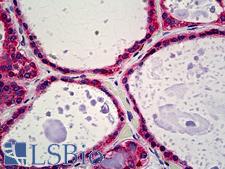
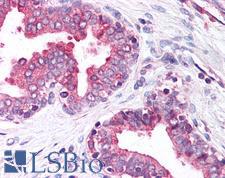
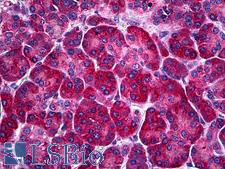
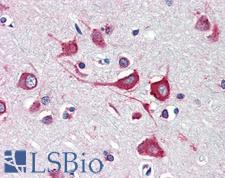
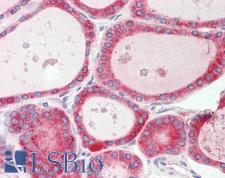
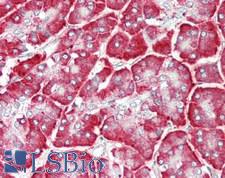
PathPlusTM CALR / Calreticulin Antibodies
Calreticulin (CALR) is a multifunctional protein that acts as a major Ca(2+)-binding (storage) protein in the lumen of the endoplasmic reticulum. It is also found in the nucleus, suggesting that it may have a role in transcription regulation. Calreticulin binds to antibodies in certain sera of systemic lupus and Sjogren patients which contain anti-Ro/SSA antibodies, it is highly conserved among species, and it is located in the endoplasmic and sarcoplasmic reticulum where it may bind calcium. Calreticulin has an important function as a modulator of nuclear hormone receptors and their regulation of gene transcription. For example, it can inhibit the glucocorticoid receptor from binding to its specific response element. It can also inhibit the binding of androgen receptor (AR) to its hormone-responsive DNA element, and can block retinoic acid receptor transcriptional activities along with retinoic acid-induced neuronal differentiation in vivo. Calreticulin is also important for the development of the central nervous system, and it is of interest in various neurological disorders where it sees deregulated expression. In immunohistochemistry, calreticulin is a secreted protein that has strong cytoplasmic/membranous positivity in the thyroid and is weakly present in most tissues throughout the body.
References: ACS Chem. Neurosci. 2019. 10 (6):2629-2646, DOI: 10.1021/acschemneuro.9b00158
6 PathPlusTM Antibodies
☰ Filters
Products
Antibodies
(6)
Type
Primary
(6)
Target
CALR / Calreticulin
(6)
Reactivity
Human
(6)
Mouse
(2)
Rat
(2)
Bovine
(1)
Dog
(1)
Hamster
(1)
Monkey
(1)
Primate
(1)
Application
IHC
(6)
IHC-P
(6)
WB
(5)
ELISA
(1)
IF
(1)
IP
(2)
Host
rabbit
(3)
mouse
(2)
sheep
(1)
Product Group
PathPlus Neuro
(6)
Isotype
IgG1
(1)
IgG1,k
(1)
Clonality
monoclonal mc
(2)
polyclonal pc
(4)
Clone
1G11-1A9
(1)
5F12-B10
(1)
Format
Unconjugated
(6)
Epitope
aa301-350
(1)
aa52-101
(1)
Publications
No
(6)

Neuroscience
CALR / Calreticulin Sheep anti-Human Polyclonal Antibody
Mouse, Dog, Rat, Human
IF, IHC, IHC-P, IP, WB
Unconjugated
125 µg/$375

Neuroscience
CALR / Calreticulin Rabbit anti-Mouse Polyclonal Antibody
Mouse, Bovine, Rat, Hamster, Primate, Human
IHC, IHC-P
Unconjugated
0.1 ml/$375

Neuroscience
CALR / Calreticulin Mouse anti-Human Monoclonal (1G11-1A9) Antibody
Human
ELISA, IHC, IHC-P, WB
Unconjugated
50 µg/$375

Neuroscience
CALR / Calreticulin Mouse anti-Human Monoclonal (5F12-B10) Antibody
Human, Monkey
IHC, IHC-P, IP, WB
Unconjugated
50 µg/$375

Neuroscience
CALR / Calreticulin Rabbit anti-Human Polyclonal (aa52-101) Antibody
Human
IHC, IHC-P, WB
Unconjugated
100 µl/$375

Neuroscience
CALR / Calreticulin Rabbit anti-Human Polyclonal (aa301-350) Antibody
Human
IHC, IHC-P, WB
Unconjugated
100 µl/$375
Viewing 1-6
of 6
product results











