Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
Products
Antibodies
ELISA and Assay Kits
Research Areas
Infectious Disease
Resources
Purchasing
Reference Material
Contact Us
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
PathPlusTM ADAM10 Antibodies
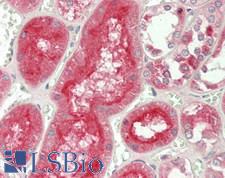
ADAM10 (A disintegrin and metalloproteinase domain-containing protein 10, CD156c) is a cell surface protein that is responsible for the proteolytic release of other cell-surface proteins on endothelial cells, including soluble JAM3, heparin-binding epidermal growth-like factor, TNF-alpha, E-cadherin and ephrin-A2. It is further responsible for the constitutive and regulated alpha-secretase cleavage of amyloid precursor protein (APP) and contributes to the normal cleavage of the cellular prion protein. It also has vesicle-based protease activity. ADAM10 also regulates the proteolytic processing of Notch and mediates lateral inhibition during neurogenesis. Increased expression of this protein in hippocampal neuronal synapses is found in the brains of Alzheimer’s disease patients. In breast cancer, inhibitors that target ADAM10 used in conjunction with Herceptin may be an effective therapy for some HER2-upregulated patients. In immunohistochemistry, ADAM10 has membranous and cytoplasmic staining in a variety of tissues including the brain, thyroid, gastrointestinal tract, liver, kidney, reproductive tissues, skin and immune tissues.
References: Cold Spring Harbor Perspectives in Medicine. 2 (5): a006270, PMID: 22553493; Cancer Biology & Therapy. 5 (6): 657–64, PMID: 16627989; PLoS One. 2016 May 25;11(5):e0156184, PMID: 27224017;
1 PathPlusTM Antibody
☰ Filters
Products
Antibodies
(1)
Type
Primary
(1)
Target
ADAM10
(1)
Reactivity
Human
(1)
Application
IHC
(1)
IHC-P
(1)
WB
(1)
Host
rabbit
(1)
Product Group
PathPlus Neuro
(1)
Clonality
polyclonal pc
(1)
Format
Unconjugated
(1)
Epitope
aa420-641
(1)
Publications
No
(1)

Neuroscience
ADAM10 Rabbit anti-Human Polyclonal (aa420-641) Antibody
Human
IHC, IHC-P, WB
Unconjugated
50 µl/$375
Viewing 1-1
of 1
product results











