SARS-CoV-2 and COVID-19 Pathogenesis: A Review
Glenna Burmer, M.D., Ph.D., Chief Scientific Officer
Mark Burmer and Vagmita Pabuwal, Division of Bioinformatics
LifeSpan BioSciences, Inc.
Introduction
On December 31, 2019, the China Health Authority notified the World Health Organization (WHO) of several cases of an unusual viral pneumonia in Wuhan City in Hubei Province in Central China. By January 7, 2020, a novel coronavirus (SARS-CoV-2) was identified as the pathogen, and the disease was named COVID-19 by the WHO. The mortality rate as of February 4, 2020, was 2.1% within China, and among hospitalized patients, it ranged between 11-15%, making it substantially more lethal than the seasonal flu, with a lower mortality rate than SARS or MERS (Harapan et al., 2020; Wang et al., 2020). Since its discovery, the pathogen’s sequence has been determined, although the pathogenesis of the disease is still under active investigation.
Biology and Life Cycle of Coronaviruses
Coronaviruses are enveloped, single-stranded positive sense RNA viruses that infect a wide variety of animal species and cause respiratory, gastrointestinal, and central nervous system diseases (Li et al., 2006; Perlman and Netland, 2009; Fehr and Perlman, 2015; Li, 2015). Coronaviruses have been subdivided into four genera based upon genetic clustering and antigenicity: alpha, beta, gamma, and delta coronaviruses (Dhama et al., 2014; Coleman et al., 2014). Common human coronaviruses include the 229E and NL63 alpha coronaviruses and the OC43 and HKU-1 beta coronaviruses. In infected humans, they are associated with a range of cold-like symptoms as well as severe respiratory tract infections (Fielding, 2011). Other more symptomatically severe human coronaviruses that have been transmitted from animals include MERS-CoV, a beta coronavirus that causes Middle East Respiratory Syndrome (MERS); SARS-CoV, a beta coronavirus that causes severe acute respiratory syndrome (SARS); and SARS-CoV-2, a beta coronavirus that causes coronavirus disease 2019 (COVID-19).
Features that are in common among these groups of coronaviruses are their large RNA genomes (26-35 kb), a highly conserved genomic organization with a large replicase gene that precedes the structural and accessory genes, expression of non-structural genes by ribosomal frameshifting, unique enzymatic activities encoded within the replicase polyprotein, and expression of downstream genes by synthesis of 3’ nested mRNAs (Fehr and Perlman, 2015).
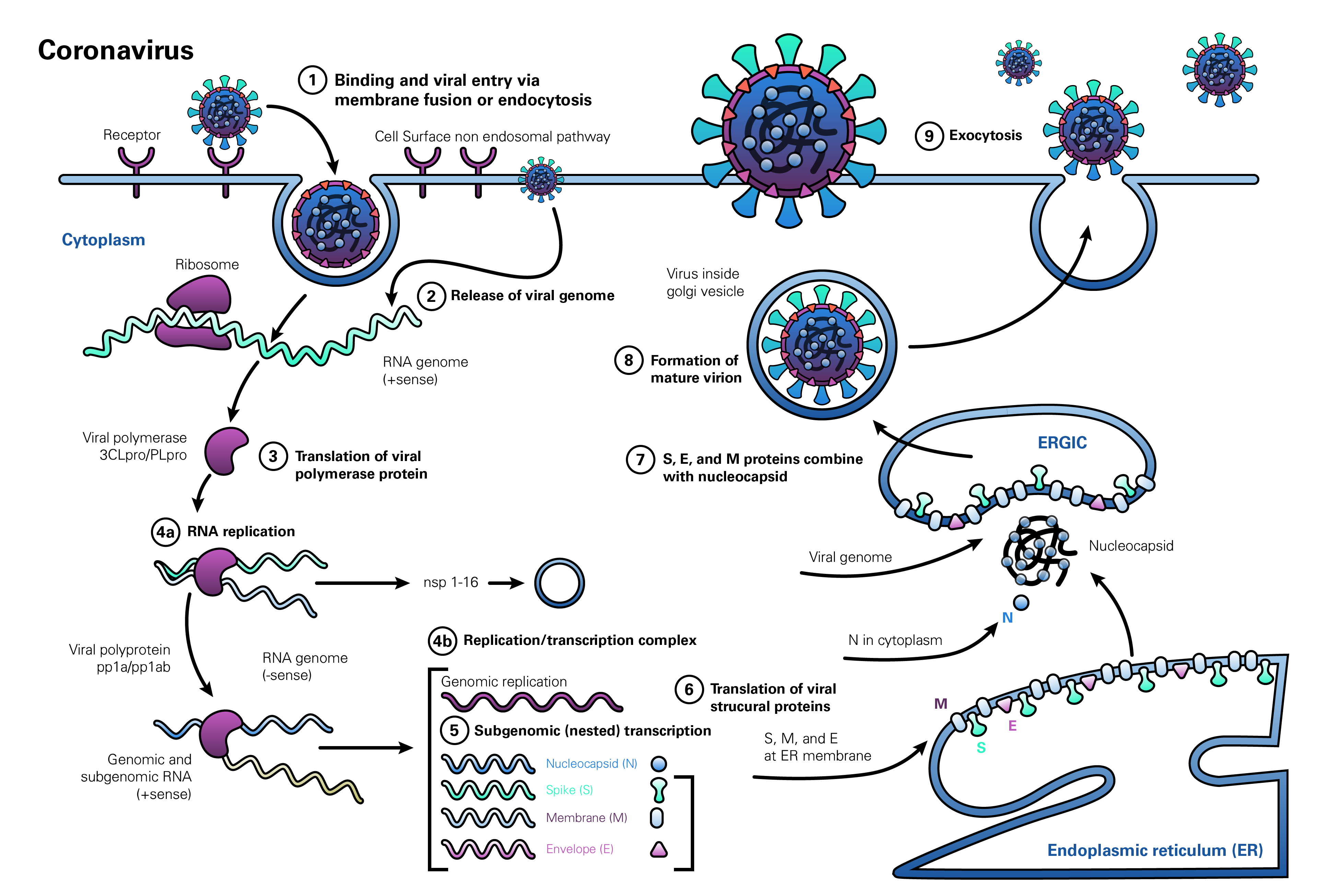
The life cycle of coronaviruses begins when the virion binds to the host cell receptor via its spike protein S1 subunit (Figure 1, step 1). The S-protein-receptor interaction determines the host species range and tissue tropism for the virus. For example, many alpha-coronaviruses use aminopeptidase N as their receptor, whereas SARS-CoV and HCoV-NL63 utilize angiotensin-converting enzyme 2 (ACE2) as the host receptor. MHV utilizes CEACAM1, and MERS-CoV binds dipeptidyl-peptidase 4 (DPP4) to enter human cells. The resulting disease profile depends to a great extent on the distribution of the receptor within tissues in the human body (Hamming et al., 2004; Fehr and Perlman, 2015; Li M-Y et al., 2020).
After receptor binding, the virus gains access to the cytosol by acid-dependent proteolytic cleavage of the S protein into S1 and S2 subunits by a furin, cathepsin, TMPRSS2, or another protease, followed by S2-assisted fusion of the viral and cellular membranes. After release of the viral genome (Figure 1, step 2), the replicase is translated from the genomic RNA (Figure 1, step 3). Viral RNA synthesis then follows (Figure 1, step 4a), with the assembly of viral replication-transcription complexes (Figure 1, step 4b). Viral structural proteins (S, E, and M) are translated from the RNA (Figure 1, step 5), inserted into the endoplasmic reticulum (Figure 1, step 6), and move to the endoplasmic reticulum-Golgi intermediate compartment (ERGIC). Multiple copies of the nucleocapsid (N protein) package genomic RNA into helical structures (riboncleoprotein complexes) in the cytoplasm, and interact with hydrophobic M proteins (envelope protein) in the ERGIC that serve to direct assembly of the virion (Figure 1, step 7). Virions budded from the membranes of the ERGIC (Figure 1, step 8) are then transported via the constitutive exocytic pathway out of the cell (Figure 1, step 9) (deHann and Rottier, 2005; Fehr and Perlman, 2015).
Structure, Phylogeny, and Genomics of SARS-CoV-2
SARS-CoV-2 has been identified as a member of the betacoronavirus family with close sequence similarity (80-96%) to the bat SARS coronavirus. A single-stranded RNA virus, SARS-CoV2 is 79% homologous to SARS-CoV, the causative agent of SARS, and uses the same angiotensin converting enzyme 2 (ACE2) as its receptor. Similar to SARS-CoV, the novel coronavirus targets cells using its spike (S) protein, although SARS-CoV-2 targets the receptor with ten times the affinity of SARS-CoV, which may explain both the faster rate of transmission and infection. The SARS-CoV-2 coronavirus genome has approximately 96% overall sequence identity with bat coronavirus RaTG13 and approximately 80% overlap with SARS-CoV. Phylogenetic analysis groups SARS-CoV-2 with betacoronavirus genus lineage B, placing it alongside a number of bat coronaviruses as well as SARS-CoV (Jaimes et al., 2020).
A look at the protein sequence of the SARS-CoV-2 spike protein reveals details about the evolution of this virus and also highlights potential pan-coronavirus targetable regions for future antibody and drug development. The spike protein sequence has homologies of 93.1% with bat coronavirus RaTG13 spike protein (Lan et al., 2020), 76% with SARS-CoV spike glycoprotein, and 71% with the bat coronavirus BM48-31/BGR/2008 spike protein, after which identity scores with other viruses drops considerably. There is generally more sequence overlap in the S2 subunit when compared to the S1 subunit.
The S1 receptor-binding domain (RBD), which is responsible for binding to the host ACE2 receptor in infected humans, has highly similar structure to the SARS-CoV S1 RBD, and their binding mode to the ACE2 receptor is almost identical (Lan et al., 2020). Overall sequence identity with SARS-CoV S1 is 66%, with bat coronavirus BM48-31/BGR/2008 S1 identity is 60%, and it has considerably less identity with most other coronaviruses’ S1 proteins. A few important additions to its sequence affect host receptor binding and cleavage activation by human proteins. Novel residues in the SARS-CoV-2 S1 RBD sequence, such as Phe486, Gln493, and Lys417, may increase its capacity to bind to ACE2 beyond that of SARS-CoV (Lan et al., 2020). Additionally, the novel “RRAR” sequence at the S1/S2 cleavage site is predicted to be a recognition sequence for the human protease furin (Hoffman et al., 2020a; Jaimes et al., 2020). This host protein may be responsible for pre-cleavage of the S protein prior to activation by TMPRSS2 in lung cells deficient in cathepsin L, another potential activation pathway. This sequence is not found in SARS-CoV or the closely related RaTG13 coronavirus, but it is present in the spike proteins expressed by MERS-CoV, HCoV-OC43, and HCoV-HKU1 (Hoffman et al., 2020b).
A prominent overlapping region across many coronavirus spike proteins is the putative fusion peptide found at the S2 subunit cleavage site, which is responsible for membrane fusion and is represented by the sequence “SFIEDLLFNKVTLADAGF” in SARS-CoV-2 (amino acids (AA) 816-833). This sequence is identical in SARS-CoV and has similarity across many other coronaviruses, including human coronavirus HKU1, MERS, TGEV, and feline infectious peritonitis virus (FIPV) (Alsaadi et al., 2019; BLAST, 2020). The internal residues “DLL” have been previously described as critical for membrane fusion (Madu et al., 2009).
There is evidence that the D614 to G614 (D614G) mutation in the SARS-CoV-2 spike protein increases infectivity of the virus. The D614G mutation, located in the C-terminal region of the S1 subunit domain, has been detected with accelerating frequency since March 2020. It is speculated to impart a transmission advantage, and analysis of SARS-CoV-2 genomes from across the globe indicates that it is now the dominant residue at position 614 (Korber et al., 2020; Wagner et al., 2020; Zhang et al., 2020). The G614 mutation is believed to be more stable than D614, and it may work to stabilize S1 and S2 subunit interactions and suppress S1 shedding. In one study, pseudoviruses carrying either the G614 mutation or original D614 genotype were produced and used to infect HEK293T cells expressing human ACE2, and the G614 genotype pseudoviruses displayed nine-fold greater efficiency at infection (Zhang et al., 2020). A greater amount of both S1 subunit and total spike protein was also observed in G614 genotype pseudoviruses, and the G614 mutation is thought to lead to an increase in spike protein incorporation into the virion. This could result in an increase in the number of binding sites and explain the potential increase in infectivity (Zhang et al., 2020). Thus far, while the G614 genotype is correlated with enhanced infectivity and an increase in viral nucleic acid in the upper respiratory tract (and likely higher viral titer), it has yet to be directly connected to increased disease severity (Korber et al., 2020; Hu J et al., 2020; Wagner et al., 2020).
Some smaller features that are preserved across a large range of coronaviruses may be of interest for the development of pan-coronavirus antibodies or vaccines, such as peptides IPTNFTISV (beta sheet, AA 714-722) and DRLITGRL (alpha helix, AA 994-1001) (PDB: 6VXX). Sequence IPTNFTISV was one of six peptides from the spike glycoprotein that was recently proposed for use in a hypothetical multi-epitope vaccine (Kalita et al., 2020). This peptide was predicted to be a cytotoxic T-cell recognition epitope of the B7 supertype and was determined to be non-toxic, antigenic, and thermostable (Kalita et al., 2020). Notably, it also has 90-100% sequence homology across feline infectious peritonitis virus, TGEV, MHV, and human coronavirus HKU1 and is present in many human papilloma viruses (BLAST). Additionally, peptide DRLITGRL, part of a helical structure near the CT end of the S2 subunit, is conserved across SARS, avian infectious bronchitis virus, human coronaviruses 229E and NL63, feline infectious peritonitis virus, and TGEV (PSI-BLAST, Uniprot: P0DTC2, PDB: 6VXX). These may be useful sequences for the development of coronavirus antibodies, drugs, or vaccines.
SARS-CoV-2 and Interspecies Transmission
Evidence of SARS-CoV-2 transmission to and between other animals has been discovered in cats, lions, tigers, minks, dogs, hamsters and ferrets (Halfmann et al., 2020; Sit et al., 2020; American Veterinary Medical Association, 2020). Cats have presented with mild symptoms or as asymptomatic in discovered cases and can show signs of gastrointestinal and respiratory disease when infected. Mink have shown low mortality rates and have also presented with gastrointestinal and respiratory symptoms (often as a cough). The sample size for domestic animals confirmed to have COVID-19 disease is small, but this may also point to fundamentally low rates of transmission of the disease from humans to pets (American Veterinary Medical Association, 2020). There is thus far no evidence of transmission from domesticated animals back to humans, and pets are not considered a cause for concern when it comes to further spread of SARS-CoV-2.
The presence of the disease in the lungs and gastrointestinal tract in pets mirrors infection patterns in humans, and it is likely that it is also infecting animal host epithelial cells by binding to ACE2. The ACE2 receptor has high conservation across common domestic and wild mammals, and moderate conservation across other classes including birds (Sun et al., 2020; BLAST, 2020). ACE2 has greater than 80% sequence identity with the same receptor found in many mammals ranging from 86% in cats to decreasing percentages in dogs (85%), rats (84%), ferrets (83%), chinchillas (84%), polar bears (83%), and orcas (81%). In birds, human ACE2 has 72% sequence identity in crows, 71% in parakeets, and 68% in pigeons.
In humans, SARS-CoV-2 gains entry to the host cell when the spike glycoprotein binds ACE2 via its S1 subunit RBD (receptor binding domain). It is known to engage the ACE2 receptor alpha-1 helix at residues H34, D30, and Q24; interact with M82 via van der Waals forces; and also utilize the alpha-2 helix and the beta-3 and beta-4 linker by forming H-bonds with residues Y41, Q42, K353, and R357 (Yan et al., 2020). Notably, Y41, Q42, K353, and R357 are conserved across human, feline, and canine ACE2. A study by Luan et al. (2020) predicted that dog, cat, pangolin, and hamster ACE2 would potentially bind the SARS-CoV-2 spike protein RBD based on sequence and structural similarity, and the virus has already been shown to infect these animals. They also concluded that rat and mouse ACE2 receptors were unlikely to bind the protein. The SARS-CoV-2 spike protein may have similar or slightly diminished binding affinity and modality compared to the ACE2 receptor expressed in non-human hosts. However, further studies are necessary to detail the exact mechanisms of SARS-CoV-2 infection in various animals.
Clinical and Pathologic Features of COVID-19
As with SARS-CoV, MERS-CoV, and influenza viruses, the primary route of disease transmission in humans is via respiratory droplets and direct contact (Guo et al., 2020; Morawaska and Cao, 2020). After a mean incubation period of 4 days, patients develop symptoms including fever, non-productive cough, sore throat, shortness of breath, headache, myalgias, and fatigue (Guan et al., 2020; Huang C et al., 2020). Unlike respiratory viruses that primarily affect the upper respiratory tree, SARS-CoV-2 infects the lower respiratory tract and alveoli, resulting in bilateral pneumonia with ground-glass opacity and patchy bilateral shadowing on computed tomography in half of patients at the time of diagnosis (Guan et al., 2020; Xu Z et al., 2020). Fifteen percent of patients present or develop severe acute respiratory disease, and of those patients, a quarter expire despite mechanical ventilation and intensive therapy for an overall case-fatality rate that ranged from 3-15% depending upon the age of the patient and the presence of existing co-morbid conditions. The cause of death is most commonly severe acute respiratory distress syndrome (ARDS), a result of direct viral pulmonary damage and the cytokine release syndrome, an uncontrolled inflammatory response resulting from the release of large amounts of pro-inflammatory cytokines and chemokines by immune cells reacting to cellular damage caused by the viral infection.
Patients also commonly presented with lymphopenia, thrombocytopenia, or leukopenia at onset of disease. The severity of the lymphopenia was predictive of poor prognosis (Huang I, 2020). Lymphocyte depletion, which is also a feature of SARS and MERS, is hypothesized to be due to a combination of factors: direct viral infection due to the presence of the ACE2 receptor on lymphocytes, release of pro-inflammatory cytokines such as IL-6, and lymphocyte sequestration (Li et al., 2004; Huang I, 2020; Lin et al., 2020).
A subset of patients also present with diarrhea, vomiting, and abdominal pain, since the ACE2 receptor is prominently located on enterocytes of the entire gastrointestinal tract, from duodenum to small intestine to the colon (Hamming et al., 2004; D’Amico et al., 2020; Wong et al., 2020). Gastrointestinal symptoms and diarrhea were also frequently observed in patients with SARS as well as MERS (Leung et al., 2003; Chan et al., 2015). Fecal-oral transmission has been implicated because SARS-CoV-2 RNA has been consistently found in biopsies of gastrointestinal tissue as well as fecal samples from infected patients (D’Amico et al., 2020; Hindson, 2020; Wong et al., 2020; Xiao et al., 2020; Xu Y et al., 2020).
The virus has also been found in the semen of infected and recovering patients (Li et al., 2020) in rectal epithelia and the oral mucosa, as well as in saliva, suggesting that the virus may also be transmitted sexually (Patri et al., 2020; Peng et al., 2020; Xu H et al., 2020).
Patients have also been reported with liver injury as detected by the presence of elevated liver enzymes, including abnormal levels of alanine and aspartate aminotransferases (ALT and AST), elevated levels of gamma-glutamyl transferase (GGT), and mild elevations in serum bilirubin. Although most of the cases had mild liver injury, the level of liver damage was proportional to the severity of COVID-19 disease (Wong et al., 2020; Zhang et al., 2020).
Neurologic manifestations have been reported in 36% of patients and were more common in patients with severe disease (Mao et al., 2020). Loss of taste (ageusia) and sense of smell (anosmia) have also been reported to be early findings in a significant percentage of patients during the early stage of COVID-19 disease (Menni et al., 2020; Speth et al., 2020; Vaira et al., 2020). Headache, dizziness, confusion, memory problems, impaired consciousness, and cases of intracerebral bleeding, viral encephalitis, and necrotizing encephalopathy have all been reported in COVID-19 patients (Cardona et al., 2020; Li YC et al., 2020; Li Z et al., 2020; reviewed by Baig, 2020). The neuroinvasive and neurotrophic properties of SARS-CoV-2 are similar to studies with other betacoronaviruses, including SARS-CoV and MERS-CoV (Li YC et al., 2020).
Pediatric patients with COVID-19 have been reported to develop a multisystem inflammatory syndrome resembling Kawasaki’s disease, with features of macrophage activation syndrome, a form of cytokine storm (Cheung et al., 2020; Verdoni et al., 2020; Whittaker et al., 2020). The patients presented with symptoms and signs such as fever, abdominal pain, conjunctivitis, rash, erythema of hands and feet, lymphadenopathy, mucous membrane changes, myocardial injury and arrhythmias, mild bilateral pneumonia, diarrhea, acute renal injury, and toxic shock. The patients tested positive for SARS-CoV-2, suggesting that coronaviruses may be responsible for other cases of Kawasaki’s disease (Verdoni et al.,2020).
Acute kidney damage has been reported in as many as 36% COVID-19 patients (Chen et al., 2020; Cheng et al., 2020; Diao et al., 2020; Guan et al., 2020; Hirsch et al., 2020; Huang C et al., 2020; Wang et al., 2020; Zhou et al., 2020). Acute kidney injury was primarily observed in patients with respiratory failure (90%), suggesting that the etiology was due to ischemic acute tubular necrosis (Hirsch et al., 2020).
ACE2 Distribution and the Pathology of COVID-19
Although the detailed mechanisms by which SARS-CoV-2 causes organ damage are unknown, the multi-system clinical presentation and progression of COVID-19 disease is consistent with direct viral injury to tissues, combined with the ensuing immunological damage triggered by the cytokine release response.
SARS-CoV-2 is known to enter cells via its spike protein binding to the ACE2 receptor on human cells (Hoffmann et al., 2020). The distribution of the ACE2 receptor in formalin-fixed human tissues has been previously reported using immunohistochemistry (IHC) with a polyclonal rabbit anti-ACE2 antibody that detected the receptor (Hamming et al., 2004). Positive staining was observed in endothelial cells in a variety of organs, arterial smooth muscle, myofibroblasts, Type I and Type II pneumocytes and respiratory epithelium of the lung, nasal and oropharyngeal non-keratinizing epithelium, enterocytes of the small intestine, skin basal layer of epidermis and hair follicles, eccrine glands, bile ducts, B and T lymphocytes and macrophages, and parietal epithelium of the kidney as well as proximal and distal convoluted tubules and collecting ducts. Staining was membranous in enterocytes, respiratory epithelium, and along the brush border of proximal tubules of the kidney. These authors reported negative staining in liver sinusoids, Kupffer cells and hepatocytes, enterocytes of the colon, and neurons and glia of the brain. They reported negative staining of the ACE2 receptor in the mesangium and glomerular endothelium of the kidney, but a recent report demonstrated endothelitis and viral elements in the endothelium of the kidney, small bowel, and lung with a prominent host mononuclear inflammatory response (Varga et al., 2020). The tissue distribution of the ACE2 receptor correlated well with the broad RNA distribution observed by PCR studies (Harmer et al., 2002; Li et al., 2020).
LifeSpan BioSciences (LSBio) has recently performed IHC on human tissues with six antibodies targeting different epitopes of ACE2 (Figure 2). In addition to the tissues previously reported positive, the ACE2 receptor is expressed on bile ductules, proximal tubules of the kidney, seminiferous epithelium of the testis, thyroid follicular epithelium, cardiac myocytes and Purkinje fibers of the conduction system, and the olfactory mucosa. In contrast to the prior study, with four antibodies to different epitopes of ACE2, staining was observed in neurons and glia within the cerebral cortex and cerebellum, in addition to capillary endothelial cells and perivascular macrophages. Endothelial cells, hepatocytes, and Kupffer cells were also positive in the liver. The widespread distribution of the ACE2 receptor may help to explain multi-organ presentation and progression of the disease.
Distribution of ACE2 in Human Tissues by Immunohistochemistry (IHC)
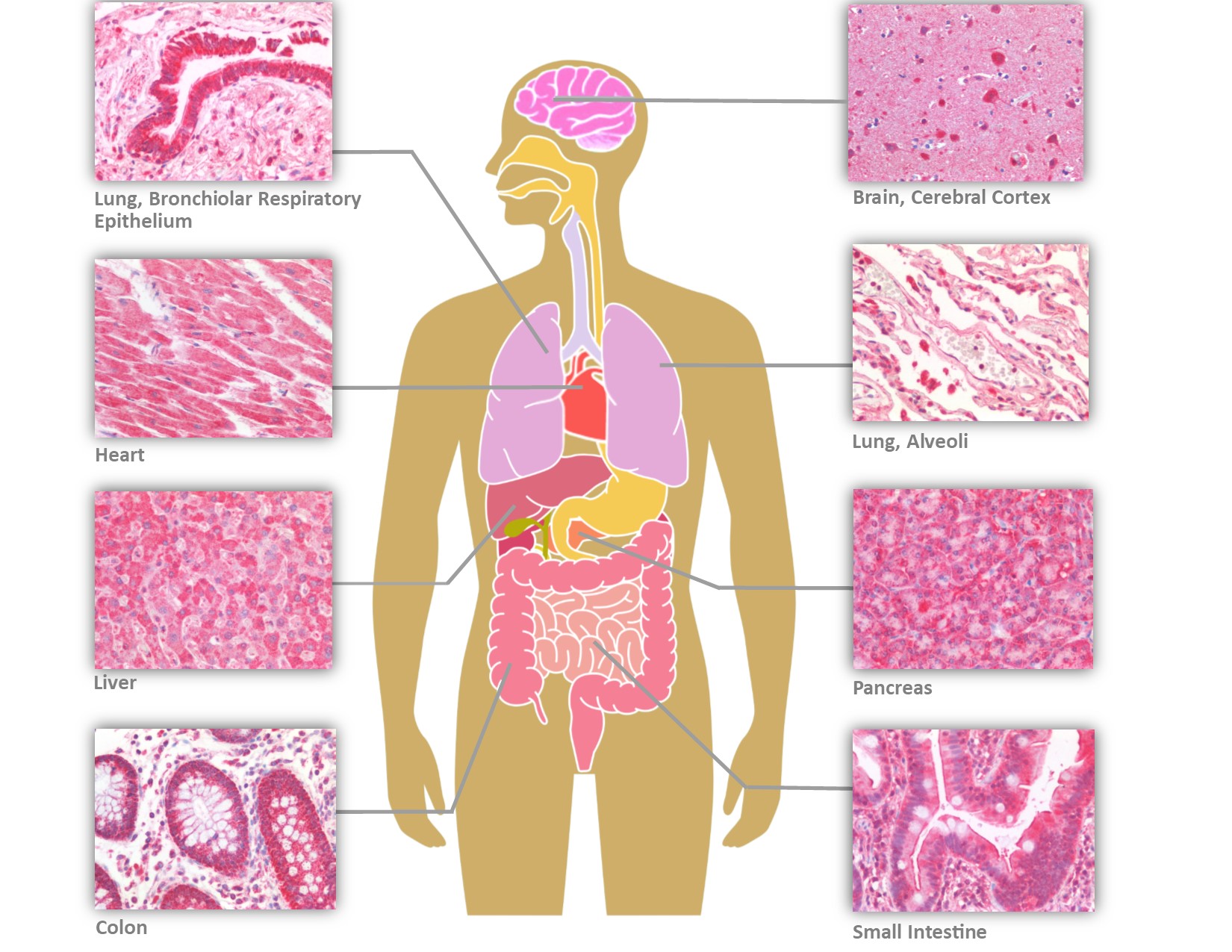
The primary portal of entry is the nasopharynx, and the virus can bind to both non-keratinizing squamous epithelium as well as to respiratory epithelium of the nasopharynx and oropharynx via its spike protein. Within the lower respiratory tract, ACE2 expression is prominent in epithelial cells lining the bronchial tree, as well as within alveolar Type I and Type II pneumocytes. As with SARS-CoV, the expression pattern helps to explain the diffuse alveolar damage and bilateral pneumonia, with CT scans showing ground-glass opacity, consolidation, and septal and pleural thickening (Hamming et al., 2004; Shi H et al., 2020).
A subset of patients also present with diarrhea, and ACE2 is prominently located on enterocytes of the entire gastrointestinal tract, from small intestine to the colon. Fecal-oral transmission has been implicated because SARS-CoV2 RNA has been consistently found in biopsies of gastrointestinal tissue as well as fecal samples from infected patients.
Despite the widespread localization of ACE2, there is restricted organ damage compared to the number of tissues that express the receptor, which suggests that a second receptor may be required for viral internalization or activation. In the case of SARS-CoV2, TMPRSS2 has been shown to be required for S protein priming and viral entry (Hoffman et al., 2020a).
Post-mortem studies on patients who expired from COVID-19 have also demonstrated the widespread nature of tissue damage (Carsana et al., 2020). Similar to findings with SARS-CoV and MERS-CoV, the authors observed diffuse alveolar damage with viral particles within pneumocytes, hyaline membrane formation, pneumocyte hyperplasia, and the presence of platelet-fibrin thrombi in small arterial vessels, and lung infarcts, indicating the presence of a coagulopathy (Carsana et al., 2020; Remmelink et al., 2020). In other organs, myocardial infarctions and ischemic enteritis were found (Remmelink et al., 2020). Autopsies on patients from New Orleans also showed acute respiratory distress syndrome, but also with areas of hemorrhage and evidence of thrombi in the lungs (Fox et al., 2020). Cardiac findings showed individual myocardiocyte necrosis without a significant lymphocytic myocarditis consistent with capillary or microvascular dysfunction, similar to the endotheliitis previously reported in COVID-19 patients (Varga et al., 2020). The widespread presence of injury and the ability of the virus to infect endothelial cells in the microcirculation of many organs may account for the multi-organ damage observed in late stage COVID-19 patients.
Cytokine Release Syndrome
A prominent feature of severe cases of COVID-19 involve a hyperimmune response to viral infection, known as Cytokine Release Syndrome (Cytokine Storm). Cytokines are low molecular weight proteins produced in the body to mediate cell signaling and regulation of the immune system. Cytokine Release Syndrome is characterized by an immune over-response, where the majority of the damage done to the host is due to an attack by the immune system rather than a direct cytotoxic effect by the pathogen. This systemic inflammatory response is mediated by the release, by immune cells, of inflammatory cytokines such asIFN-alpha, IFN-gamma, IL-1, IL-1beta, IL-2, IL-2R, IL-6, IL-8, IL-10, IL-12, IL-17D, IL-18, IL-33, TNF-alpha, TGF-beta, and chemokines such as CCL2, CCL3, CCL5, CXCL8, CXCL9, and CXCL10. The overproduction of inflammatory cytokines leads to an attack on multiple organ systems, resulting in ARDS, multi-organ failure, and hypotensive shock (Huang et al., 2020; Li X et al., 2020).
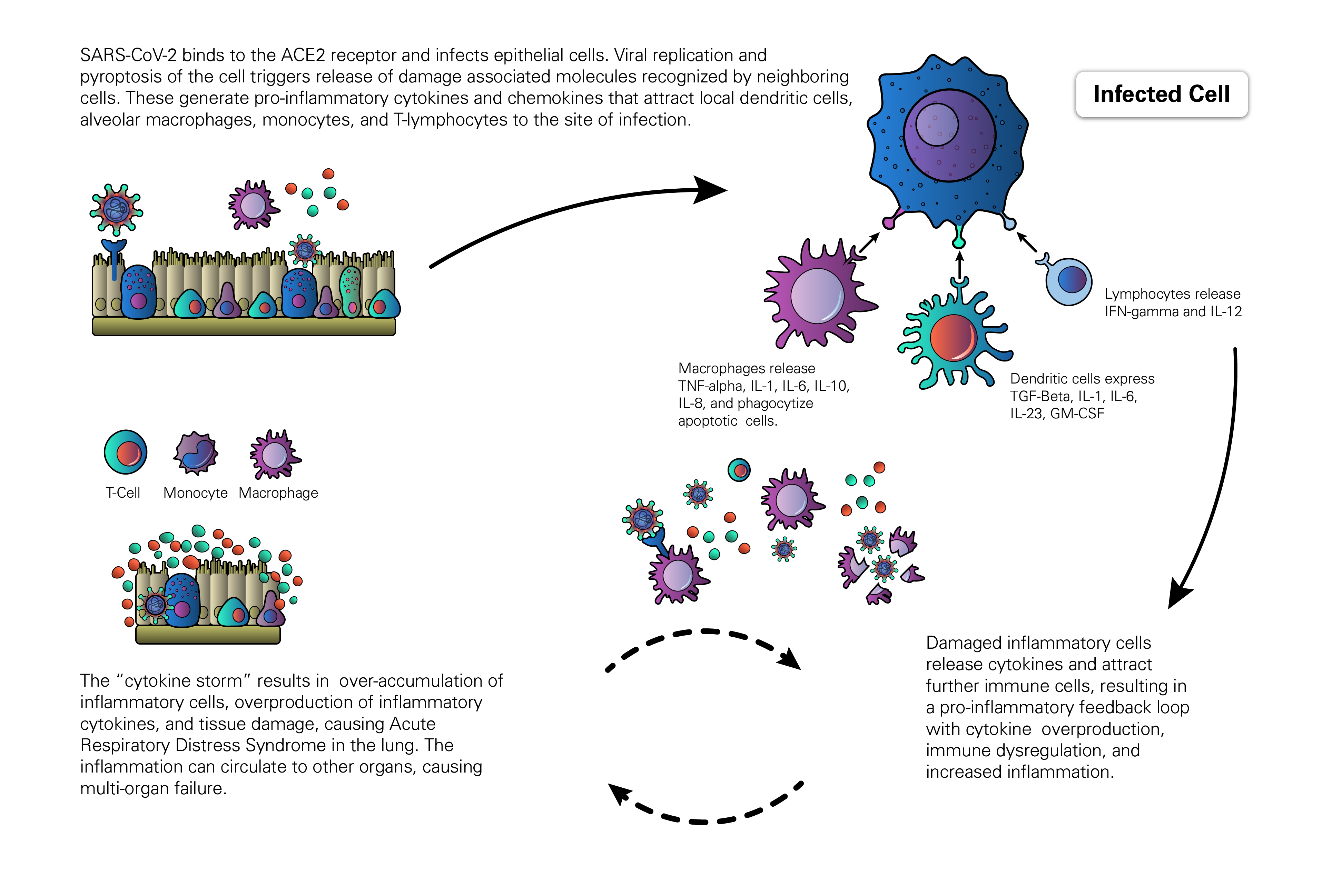
The host response to SARS-CoV-2 begins after the virus binds epithelial cells in the nasopharynx, bronchiolar tree, and alveoli of the lung. Studies with SARS-CoV found that epithelial cells respond to viral invasion by secreting molecules such as IL6, IL-8, and IP-10 to initiate and sustain inflammatory responses. Similarly, macrophages and dendritic cells that reside at sites of infection process viral antigens, engage in phagocytosis, and produce a broader array of cytokines to relay and amplify the inflammatory signals initiated by the virally-infected epithelial cells (Yoshikawa et al., 2009). As cytokines are released, they drive a positive feedback response in which immune cells are recruited to sites of inflammation and continue the autoimmune attack, furthering damage of both infected and uninfected cells. As the viral infection progresses to other organs and infects both epithelial and endothelial cells, the activation of the innate immune response results in further release of pro-inflammatory cytokines as well as activation of the complement system. The activation of the complement cascade further increases vascular permeability and promotes the rapid spread of inflammation (Tanaka et al., 2016).
Both severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome CoV (MERS-CoV) cause a severe and highly lethal respiratory disease in humans that is also characterized by a prominent pro-inflammatory response and mediated by release of cytokines (Chan et al., 2015).
Diagnostic Test Kits for SARS-CoV-2
The clinical diagnosis of SARS-CoV-2 is based upon clinical manifestations and radiographic or chest CT scans that show bilateral pneumonia. However, rapid diagnostic quantitative RT-PCR tests are now available to detect viral RNA present in nasal or oropharyngeal swabs. Viral RNA is released from virions present in the nasopharyngeal or oropharynx mucosa a few days after infection. These kits detect the nucleocapsid (N) or ORF1ab regions of the viral RNA genome. These tests tend to be highly sensitive and specific, although false negative PCR results can occur due to inadequate sampling (Corman et al., 2020). Furthermore, these tests require advanced training, expensive equipment, and must be performed in a BL-2 containment laboratory that has experience handling infectious pathogens.
An easier, more rapid test to determine if a patient has developed an antibody response to the virus is a human SARS-CoV-2 IgG/IgM antibody detection kit. Approximately 2 weeks after primary infection, patients begin to develop detectable IgM and IgG responses to SARS-CoV-2. The presence of anti-viral antibodies can be detected by a lateral flow detection kit based upon colloidal gold immunochromatography. These kits use a combination of colloidal gold-coated SARS-CoV-2 antigen and anti-human IgG and IgM antibodies to bind to the anti-SARS-CoV-2 antibodies present in patient serum or blood samples, creating colored lines if the patient tests positive. These kits are sensitive, specific, and rapid (less than ten minutes), and require only a few microliters of blood via fingerstick to perform the assay. Because patient samples can be infectious, these tests are also performed by clinical healthcare professionals in a controlled laboratory setting.
Treatment of SARS-CoV-2 Infection and COVID-19 Disease
The treatment of COVID-19 disease is based upon a multi-pronged approach wherein biochemicals are administered to target different stages of the viral replication cycle, combined with modulating the host’s immune response to the infection. Compounds that interfere with the viral life cycle include those that block viral entry, directly inhibit replication, or suppress viral exocytosis. Compounds that interfere with host proteins that are otherwise co-opted by the virus for replication and infection may also have therapeutic potential. These include proteins such as the SARS-CoV-2 spike protein receptor ACE2, protease TMPRSS2, and casein kinase II (CK2) (Bouhaddou et al., 2020; Hoffman et al., 2020a). Furthermore, once the infection is established, immunomodulators such as JAK inhibitors may be helpful in ameliorating or counteracting the immune dysregulation and cytokine release syndrome that contributes to severe COVID-19 disease.
Inhibitors of the viral life cycle include those that block viral entry, replication, assembly, or release. A number of compounds are predicted to inhibit SARS-CoV-2 viral entry into the host cell, including camostat mesylate and nafamostat mesylate. TMPRSS2 is a human protease used by SARS-CoV-2 for spike protein priming during host cell entry, and inhibition of TMPRSS2 with camostat mesylate or nafamostat mesylate has been shown to block SARS-CoV-2 infection in lung cell lines and Vero E6 cells (Hoffman et al., 2020a; McKee et al., 2020). Other compounds that are predicted to interfere with spike protein binding, membrane fusion, and viral entry include toremifene, emodin, soluble ACE2 (hrsACE2), umifenovir, nitazoxanide, and hydroxychloroquine (Dyall et al., 2017; Cheng and Martin, 2020; Li G et al., 2020; McKee et al., 2020; Monteil et al., 2020; Wang Z et al., 2020; Zhou et al., 2020).
Another potential approach is to target viral replication in cells that have already been infected with the virus. Nucleoside analogs are of great interest as a means of suppressing viral replication by interfering with the ability of viral RNA-dependent RNA polymerase (RdRp) to properly synthesize viral RNA. RdRp is pivotal for SARS-CoV-2 genomic replication, and nucleoside analogues that are designed to be incorporated into viral RNA by RdRp can create deleterious mutations during RNA synthesis that produce premature chain termination (Warren et al., 2016; McKee et al., 2020; Shannon et al., 2020). Remdesivir, favipiravir, galidesivir, and EIDD-1931 are among the nucleoside/ribonucleoside analogs which have been shown to directly inhibit SARS-CoV-2 replication and reduce overall viral titers (Elfiky, 2020; McKee et al., 2020; Shannon et al., 2020; Sheahan et al., 2020; Wang M, 2020; Yin et al., 2020).
Viral replication and assembly can also be suppressed by targeting various structural and non-structural proteins that are vital for the viral life cycle. For example, the SARS-CoV-2 proteases Mpro and PLpro are important for processing viral polyproteins and for viral replication. The compounds ebselen and nelfinavir have been shown to inhibit the activity of these proteases, resulting in a reduction in viral replication when tested in Vero cells (Jin et al., 2020; McKee et al., 2020; Węglarz-Tomczak et al., 2020; Xu Z et al., 2020). Famotidine has also been shown to inhibit the Mpro protease, and is being evaluated in a number of clinical trials to determine its utility in the treatment of COVID-19 disease (Freedberg et al., 2020; Clinical trials NCT04370262 and NCT04389567). Separately, compounds such as the natural polyphenol resveratrol, which is believed to inhibit SARS-CoV-2 nucleocapsid expression, and the NSAID indomethacin, which may interfere with SARS-CoV-2 Nsp7 protein activity, may be useful in suppressing SARS-CoV-2 infection (Horne and Vohl, 2020; McKee et al., 2020; Li and DeClercq, 2020; Xu Z et al., 2020; Zhang and Liu, 2020).
In addition to directly or indirectly targeting various processes in the SARS-CoV-2 viral life cycle, immunomodulatory drugs are of interest in counteracting the damaging effects of the immune dysregulation that arises in COVID-19 disease. Various clinical trials are currently underway to evaluate drugs that interfere with pro-inflammatory cytokine signaling pathways in an attempt to increase survival in patients with severe disease. A few prominent examples include tocilizumab, ruxolitinib, baricitinib, fedratinib, and dexamethasone.
A more detailed description of each of these inhibitors is given in the Table provided below.
Table of Potential Therapeutics to Treat COVID-19
| Class of Therapeutic | Name | Mechanism of Action |
|---|---|---|
| Viral Life Cycle Inhibitors | Camostat Mesylate | Inhibits serine protease TMPRSS2, required for SARS-CoV-2 Spike protein priming |
| Nafamostat Mesylate | Inhibits serine protease TMPRSS2, required for SARS-CoV-2 Spike protein priming | |
| hrsACE2 | Soluble ACE2 blocks viral attachment to host ACE2 protein | |
| Hydroxychloroquine | Increase pH in fusion vesicles, inhibit spike protein binding to receptor, immune modulator | |
| Toremifene, Emodin | Block fusion of viral and endosomal membranes, inhibits Spike protein and host cell entry | |
| Umifenovir (Arbidol) | Block clathrin-mediated endocytosis, confined particles in clathrin-coated vesicles | |
| Nitazoxanide | Blocks viral host cell entry; blocks viral replication | |
| Remdesivir | Nucleoside analogue chain terminator inhibits viral replication | |
| Favipiravir (Avigan) | Nucleoside analogue chain terminator inhibits viral replication | |
| Galidesivir | Adenosine analogue RNA chain terminator | |
| EIDD-1931 | Ribonucleoside analogue induces mutations in RNA virions | |
| N3 | Inhibits serine protease TMPRSS2, required for SARS-CoV-2 Spike protein priming | |
| Ebselen | Inhibits Plpro and Mpro proteases | |
| Nelfinavir | Inhibits Mpro protease; inhibits inflammatory cytokines | |
| Famotidine | Inhibits Mpro (3CLpro) protein | |
| Resveretrol | Inhibits nucleocapsid expression, increases ACE2 expression | |
| Indomethacin | Interacts with Nsp7 protein, blocks RNA synthesis; anti-inflammatory | |
| Immunomodulators | Tocilizumab | Monoclonal antibody inhibits IL-6 |
| Ruxolitinib | JAK1 and JAK2 inhibitors suppress cytokine release, inhibits MARK1 and MARK3 interaction with Orf9b | |
| Baricitinib | JAK kinase inhibitor suppresses cytokine release; blocks viral endocytosis | |
| Fedratinib | JAK2 kinase inhibitor suppresses cytokine release and production of IL-17 and IL-17F |
Preventing SARS-CoV-2 viral entry
Camostat Mesylate
Camostat mesylate (CAS number 59721-28-7) is an inhibitor of the protease TMPRSS2, originally developed to treat diseases such as oral squamous cell carcinoma and chronic pancreatitis (McKee et al., 2020). TMPRSS2 is used by SARS-CoV-2 for spike protein priming during host cell entry, and inhibition of TMPRSS2 with camostat mesylate has been shown to block SARS-CoV-2 infection in lung cell lines. Furthermore, studies have indicated that camostat mesylate usage over multiple weeks is likely to be well tolerated, and thus it is of interest in the treatment of COVID-19 disease (Hoffman et al., 2020b).
Nafamostat Mesylate
Nafamostat mesylate (CAS number 81525-10-2, 82956-11-4) is another serine protease inhibitor previously shown to inhibit MERS-CoV spike protein fusion to host cells by blocking TMPRSS2 activity. It has been shown to suppress SARS-CoV-2 infection in simian Vero E6 cells and may be an effective agent against SARS-CoV-2 infection (McKee et al., 2020).
hrsACE2
There is evidence that the administration of soluble human recombinant ACE2 (hrsACE2) protein may be an effective way to suppress SARS-CoV-2 in the early stages of infection. Monteil et al. (2020) demonstrated that hrsACE2 was able to block SARS-CoV-2 attachment to host cells and inhibit viral replication in a dose-dependent manner when tested in infected Vero cells and engineered human organoids.
Targeting Viral Membrane Fusion and Replication
Chloroquine and Hydroxychloroquine
Chloroquine is an antimalarial and immunomodulatory drug that is used to treat rheumatoid arthritis and auto-immune diseases. It acts as a lysosomotropic agent that accumulates inside lysosomes and other intracellular compartments, functioning to reduce the acidity of these vesicles and inhibit autophagic processes (Villamil Giraldo et al., 2014; Savitz and Styka, 2020). Hydrochloroquine is a derivative of chloroquine and is considered to be less toxic. There is evidence that these compounds may exert direct antiviral effects on the Zika and Ebola viruses, and they are of interest in the treatment of COVID-19 disease (Delvecchio et al., 2016; Liu et al., 2020). Increasing the pH in intracellular vesicles may interfere with viral fusion and replication. It may also alter ACE2 glycosylation in a manner that inhibits SARS-CoV-2 spike protein binding to the receptor (Devaux et al., 2020). Furthermore, hydroxychloroquine is of therapeutic interest as a potential means to decrease the inflammatory response and the risk for a cytokine storm in infected patients, as it has anti-inflammatory capacity and has been found to decrease the production of cytokines and other pro-inflammatory factors (Liu et al., 2020). A number of randomized clinical trials are currently evaluating the efficacy of administering chloroquine and hydroxychloroquine as part of COVID-19 disease treatment (Pastick et al., 2020). There have been concerns over the side effects resulting from prolonged usage of these drugs, which include higher risk for cardiac arrhythmias and QT interval prolongation (Mercuro et al., 2020). Preliminary research has shown conflicting results, with some studies determining that chloroquine is effective in limiting SARS-CoV-2 replication, and others concluding that administration does not lead to improvement or leads to a higher incidence of adverse events (Cortegiani et al., 2020; Geleris et al., 2020; Kearney, 2020; Tang et al., 2020). Further clinical trials will be necessary to prove their efficacy or lack thereof in the treatment of COVID-19 disease.
Toremifene and Emodin
Toremifene is a nonsteroidal selective estrogen receptor modulator (SERM) used in the treatment of metastatic breast cancer. It has been found to block the fusion of viral and endosomal membranes by destabilizing viral membrane glycoproteins, and has been demonstrated to inhibit Ebola, MERS-CoV and SARS-CoV viral replication via in vitro assays using established cell lines (Dyall et al., 2017; Zhou et al., 2020). There is evidence that toremifene inhibits SARS-CoV-2 Spike (S) protein and may also interact with NSP14, and it is thus of interest as a potential means of interfering with SARS-CoV-2 host cell entry and viral replication (Cheng and Martin, 2020). A drug network analysis study has proposed the repurposing of toremifene along with the compound emodin for use against SARS-CoV-2 infection. Emodin is an anthraquinone derivative that inhibits the SARS-CoV ORF3a protein and blocks interaction between ACE2 and the SARS-CoV spike protein. These compounds would simultaneously target separate pathways in the network of human-coronavirus protein interactions, and together may be an effective means of inhibiting SARS-CoV-2 infection (Zhou et al., 2020).
Umifenovir
Umifenovir (Arbidol) is a broad-spectrum antiviral that inhibits viral entry by blocking membrane fusion. In viruses that use clathrin-mediated endocytosis as a means of host cell entry, umifenovir interferes with this process by inhibiting membrane scission. It further induces the accumulation and confinement of viral particles in clathrin-coated vesicles. It is able to prevent viruses such as Ebola, hepatitis C, and influenza A and B from entering host cells (Boriskin et al., 2006; Blaising et al., 2013; McKee et al., 2020). An initial pilot trial found that administration of umifenovir was effective at reducing viral titer in patients infected with SARS-CoV-2 (McKee et al., 2020; Wang Z et al., 2020), and more clinical trials are currently underway (Clinical trials NCT04260594, NCT04286503, NCT04273763, NCT04350684).
Nitazoxanide
Nitazoxanide is an antiprotozoal anthelmintic compound approved for use in the treatment of diarrhea (Li G et al., 2020). Nitazoxanide and its active metabolite tizoxanide have been demonstrated to have antiviral activity and inhibit replication of a broad range of viruses, including hepatitis B and C, rotaviruses, MERS and other coronaviruses, noroviruses, dengue, yellow fever, Japanese encephalitis, HIV, and parainfluenza and influenza viruses (Rossignol, 2016). It was shown to inhibit SARS-CoV-2 at a low concentration in Vero E6 cells infected with the virus (Rajoli et al., 2020; Rossignol, 2016; Wang M et al., 2020). Nitazoxanide is also able to suppress influenza by interfering with N-glycosylation of the influenza hemagglutinin protein. It is possible that it has similar action against the SARS-CoV-2 spike protein and thus functions by impacting host cell entry. This is as yet undetermined, and the details of its effects on SARS-CoV-2 will require further research (Rajoli et al., 2020). A number of clinical studies are currently evaluating its safety and utility in the treatment of COVID-19 disease alone or alongside other antiviral compounds (Clinical Trials NCT04360356, NCT04361318, NCT04348409, NCT04359680, NCT04406246, NCT04341493).
Inhibiting polymerase activity and replication with nucleoside analogs
Remdesivir
Remdesivir is an antiviral compound that can inhibit viral replication, and the chemical and its parent nucleoside GS-441524 have together been shown to be effective against a number of RNA viruses including Ebola and feline infectious peritonitis virus (FIPV) (Murphy et al., 2018; Pederson et al., 2019). In research in cats infected with FIPV, Pederson et al. (2019) found that the GS-441524 compound was able to suppress and even reverse advanced disease. Feline infectious peritonitis normally has near 100% lethality in cats, but administration of GS-441524 resulted in a majority of infected cats recovering from the disease and achieving sustained remission well after the end of the study. Administration led to resolution of fever, jaundice, dyspnea, and thoracic effusion, and also appropriate weight gain and increased activity (Pederson et al., 2019). The effectiveness of the drug on feline coronavirus has led to increased interest in studying its utility against other coronaviruses, including SARS-CoV-2.
Remdesivir and GS-441524 may have similar utility in COVID-19 disease. Functionally, remdesivir directly inhibits viral replication by disrupting RdRp activity (RNA-dependent RNA polymerase, NSP12 in SARS-CoV-2). RdRp is pivotal for SARS-CoV-2 genomic replication, and nucleoside analogues like remdesivir are designed to be incorporated into viral RNA by RdRp. Their incorporation encourages deleterious mutations and also premature chain termination, thereby disrupting RNA synthesis (Warren et al., 2016; McKee et al., 2020; Shannon et al., 2020). Remdesivir has been demonstrated to inhibit SARS-CoV-2 replication in human cell lines at a low-micromolar concentration (Wang M et al., 2020). Multiple human clinical trials are currently underway to determine its efficacy at suppressing COVID-19 (Amirian and Levy, 2020; clinical trials NCT04292899, NCT04292730, NCT04365725).
Favipiravir
Favipiravir (Avigan, T-705) is a nucleoside analog of interest in combination drug therapies for COVID-19 disease, serving to directly inhibit viral replication. In the same manner as remdesivir, it inhibits viral (and not human) RNA synthesis by incorporating into viral RNA transcripts to induce premature termination (McKee et al., 2020; Shannon et al., 2020; Warren et al., 2016). Favipiravir has demonstrated activity against a wide range of RNA viruses and is approved for use against influenza virus in Japan. Furthermore, in a recent study it has been shown to increase viral mutation rates, suppress replication, and decrease viral titer in Vero cells infected with SARS-CoV-2 (Shannon et al., 2020). Trials are currently underway to determine its utility in COVID-19 disease and include testing its administration alone or alongside drugs that target membrane fusion or provide immunomodulatory functions such as chloroquine, tocilizumab, and interleukin-6 monoclonal antibody inhibitors (McKee et al., 2020; Clinical trials NCT04310228, NCT04336904, NCT04303299, NCT04392973, NCT04346628).
Galidesivir
Galidesivir is a broad-spectrum antiviral compound that has been found to tightly bind to the SARS-CoV-2 RdRp (RNA-dependent RNA polymerase, NSP12) (Elfiky, 2020). Like favipiravir and remdesivir, galidesivir is a nucleotide analog that incorporates into viral RNA via RdRp, resulting in non-obligate RNA chain termination and disrupted RNA synthesis (Yin et al., 2020). It has been demonstrated to inhibit SARS-CoV-2 viral replication in cell-based assays (Yin et al., 2020). Galidesivir is being evaluated in a clinical trial to determine its efficacy against COVID-19 disease (NCT03891420).
EIDD-1931
EIDD-1931, or ribonucleoside analog Beta-d-N4-hydroxycytidine, is a broad-spectrum antiviral compound that inhibits MERS-CoV, SARS-CoV, and bat coronaviruses. Administration of EIDD-2801 in mice infected with SARS-CoV or MERS-CoV resulted in reduced viral titer and better pulmonary function. There is evidence that it functions to increase the transition mutation rate to lethal levels in specifically viral genomic RNA and not host RNA. EIDD-1931 was demonstrated to inhibit SARS-CoV-2 replication in Vero E6 cells and also human airway epithelia cell cultures infected with the virus (Sheahan et al., 2020). EIDD-1931 is currently being evaluated in multiple clinical trials to assess the safety, tolerability, and effectiveness of the compound for use in COVID-19 disease (NCT04405739, NCT04405570, NCT04392219).
Inhibiting the SARS-CoV-2 Mpro and PLpro proteases
N3
The viral Mpro protease (Main protease, 3CLpro) is required for processing viral polyproteins expressed by SARS-CoV-2. It is thus a target of interest for inhibition therapies in the treatment of COVID-19 disease. N3 is a Michael acceptor inhibitor previously found to block the Mpro proteases expressed by SARS-CoV, MERS-CoV, and HCoV (NL63) (Ren et al., 2013; Wang F et al., 2016). It has recently been shown to irreversibly inhibit SARS-CoV-2 Mpro protease as well (Jin et al., 2020). It has been tested in simian Vero cells, where it showed antiviral effects against SARS-CoV-2 (Jin et al., 2020; McKee et al., 2020).
Ebselen
The SARS-CoV-2 Papain-like protease (PLpro) is required for SARS-CoV-2 viral replication and it is also involved in polyprotein processing. It is further utilized by the virus to co-opt the host’s immune system and increase viral replication and infection (Węglarz-Tomczak et al., 2020). The compound ebselen and its analogues, which have anti-inflammatory and cytoprotective activity, have been demonstrated to block both the SARS-CoV-2 PLpro and Mpro proteases and inhibit viral replication in simian Vero cells (Jin et al., 2020; McKee et al., 2020; Węglarz-Tomczak et al., 2020) and may be useful antiviral agents for the treatment of COVID-19 disease.
Nelfinavir
Nelfinavir is an HIV-1 protease inhibitor that has also been demonstrated to suppress SARS-CoV viral replication (Yamamoto et al., 2004). Nelfinavir was found to inhibit the SARS-CoV-2 Main protease (Mpro, 3CLpro) when tested in Vero E6 cells (Xu Z et al., 2020). Furthermore, it inhibits inflammatory cytokines in vitro and may be effective at reducing the inflammatory response and severe innate immune activation resulting from COVID-19 and other viral diseases (Wallet et al., 2012; Xu Z et al., 2020).
Famotidine
Famotidine is a compound that suppresses gastric acid production and also inhibits HIV replication in vitro (Bourinbaiar and Fruhstorfer, 1996; Freedberg et al., 2020). It has been computationally predicted to inhibit the SARS-CoV-2 Mpro (3CLpro) protein, and is thus of interest as an antiviral agent against the virus (Wu C et al., 2020). Furthermore, a retrospective cohort study analyzing 1,620 hospitalized COVID-19 patients found that administration of famotidine was associated with improved outcome (as reduced risk of death) (Freedberg et al., 2020). Clinical trials evaluating the administration of famotidine as a potential COVID-19 treatment include NCT04370262 and NCT04389567.
Inhibitors to other structural and non-structural SARS-CoV-2 proteins:
Resveratrol
Resveratrol is a natural polyphenol derived from red wine grape skins and peanuts, and it has been shown to inhibit MERS-CoV nucleocapsid expression, suppress infection, and improve post-infection host-cell survival (Lin et al., 2017; McKee et al., 2020). There is also evidence that it increases ACE2 expression, which may or may not aid in COVID-19 treatment (Horne and Vohl, 2020). Due to its activity against MERS-CoV, resveratrol has been identified as a compound of interest in suppressing SARS-CoV-2 infection (Horne and Vohl, 2020; Li and De Clercq, 2020; McKee et al., 2020; Zhang and Lui, 2020). It is also currently undergoing a clinical trial to determine its efficacy against the disease (Clinical Trial NCT04400890).
Indomethacin
Indomethacin is an NSAID (nonsteroidal anti-inflammatory drug) that is used to treat the heart condition Patent Ductus Arteriosus (PDA) in premature infants. It relieves pain, swelling, and joint stiffness that result from various conditions including arthritis, gout, bursitis, and tendonitis. Indomethacin works to suppress the inflammatory response; it reduces prostaglandin (PG) synthesis by inhibiting cyclooxygenase and blocks the formation of painful nerve impulses in inflammatory tissues (Raaben et al., 2007; Maurice and Su, 2009). There is evidence that indomethacin interacts with the SARS-CoV-2 Nsp7 protein, and it may exhibit anti-viral activities by blocking viral RNA synthesis. A preliminary study in infected dogs has indicated that administration of indomethacin reduces SARS-CoV-2 viral titer (Xu Z et al., 2020).
Managing cytokine release syndrome with immunomodulators and JAK inhibitors
Tocilizumab
Tocilizumab is a recombinant monoclonal antibody that inhibits Interleukin 6 (IL6) activity by binding to IL-6 receptors (IL6R), and it is used as an immunosuppressant in the treatment of rheumatoid arthritis. IL-6 has pro-inflammatory effects that include regulating and increasing fever and body temperature as well as mediating reactive warning signals to emergent events in the body. It promotes the production of inflammatory cytokines in the innate immune response to pathogens and tissue damage (Tanaka et al., 2014). Tocilizumab can be used to modulate IL6-promoted inflammation by preventing its signal transduction (Sebba, 2008; Meley et al., 2017). IL-6 is found to be upregulated in patients with severe COVID-19 disease, and it is believed to play a major role in the excessive inflammation and cytokine release syndrome that can result from SARS-CoV-2 infection. In a preliminary study, administration of tocilizumab in a group of COVID-19 patients was correlated with improved recovery, lowered body temperature, better oxygenation and symptomatic relief (Xu X et al., 2020). A number of clinical trials are underway to further examine its therapeutic potential for the disease (NCT04332913, NCT04315480, NCT04320615).
Ruxolitinib
Ruxolitinib is a JAK (Janus kinase) inhibitor with potential anti-neoplastic and immunomodulating activities. It specifically binds to tyrosine kinase JAK1 and JAK2 and reduces inflammation and cellular proliferation. JAK inhibitors such as ruxolitinib are being used in trial studies as potential therapies for the cytokine release syndrome and immune dysregulation that results from COVID-19. Additionally, ruxolitinib may affect SARS-CoV-2 directly; it inhibits human MARK1 and MARK3, which putatively interact with the Orf9b protein expressed by SARS-CoV-2 (Gordon et al., 2020; Clinical Trial NCT04334044).
Baricitinib
Baricitinib is a JAK inhibitor that is used to treat rheumatoid arthritis. It is an anti-inflammatory compound that can be used to regulate the production of cytokines by suppressing JAK protein activity. It is of interest as a potential means of suppressing the acute cytokine release syndrome and immune dysregulation that occurs in patients with moderate to severe COVID-19 disease (Cantini et al., 2020). Furthermore, it has been computationally predicted to inhibit AP2-associated kinase 1 (AAK1), which promotes receptor-mediated endocytosis, a process frequently used by viruses for host cell entry. It is speculated that baricitinib might suppress SARS-CoV-2 endocytotic entry by blocking AAK1 activity (Cantini et al., 2020; Richardson et al., 2020; Spinelli et al., 2020). A recent pilot study examined the effects of baricitinib administration on patients hospitalized with COVID-19 disease characterized by moderate pneumonia. It found that the compound was well tolerated, and did not lead to adverse events in the infected patients. Furthermore, improvement in respiratory function and a reduction in fever and other symptoms were seen in the baricitinib-treated group compared to the control group (Cantini et al., 2020). Current clinical trials examining baricitinib in the context of COVID-19 disease include NCT04340232, NCT04421027, NCT04373044, NCT04362943, and NCT04393051.
Fedratinib
Fedratinib is a JAK2 inhibitor that can suppress cytokine production, including the pro-inflammatory IL-17 and IL-17F cytokines expressed by Th17 cells. It is approved for use as part of treatment for myeloproliferative neoplasms, and has been shown to lower disease severity in murine models of multiple sclerosis. Its effects at reducing Th17-cell cytokine production help reduce the damage caused by the cytokine overproduction (cytokine release syndrome) that can occur in these diseases. Fedratinib is of interest as a potential means of suppressing the immune dysregulation component of COVID-19 disease (Wu and Yang, 2020).
Transmission of SARS-CoV-2 and the Role of Personal Protective Equipment
The SARS-CoV-2 virus spreads by direct contact, either from respiratory droplets that have been inhaled or from fomites that have landed on a surface that a person has touched. These known routes of transmission have resulted in a number of protective hygiene recommendations by the WHO and CDC. These include frequent handwashing or use of alcohol-based hand sanitizers, avoiding contact with people in crowded spaces, maintaining a minimum 6-foot distance, wearing face masks, avoiding touching one’s nose and mouth, and self-isolation (https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html; https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public).
Airborne and Respiratory Transmission
The SARS-CoV-2 virus particle is 0.1 microns in diameter, but expelled fomites range in size from 1-500 microns in diameter (Bar-on et al., 2020; Morawaska and Cao, 2020). Larger droplets (>100 microns) fall at shorter distances due to gravity, but intermediate-sized droplets can be transported up to tens of meters away. Smaller droplets (<10 µm) may travel long distances with air flow and remain suspended in the air for hours or days (Morawaska and Cao, 2020; Verma et al., 2020). Although the plume of expired viral particles becomes diluted as it travels, the local concentration of particles is highly dependent upon the rate and direction of air flow surrounding the individual after expiration. In outdoor settings, the viral particles may be immediately diluted and carried away, whereas indoors the viral plume may remain within the vicinity of the individual for several hours. The virus-containing droplets that fall to a surface may also remain viable for days, even after undergoing complete evaporation and forming “droplet nuclei,” emphasizing the importance of frequent cleansing of surfaces with disinfectants (van Doremalen et al., 2020; https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations).
Because of the tendency for viral droplets to stay airborne, large clusters of infection may occur, particularly when individuals are in a confined space in close proximity to one another. Precautions, therefore, should include avoiding air recirculation, avoiding close contact within another person’s exhalations, maximizing natural ventilation, and decreasing the density of people who are in contact within a space. Also, the wearing of personal protective equipment, particularly masks and respirators, can be protective in public spaces.
An uncovered cough can travel 12 feet within 50 seconds, much farther than the currently recommended 6-foot distancing guidelines, and so face masks become an important to minimizing the risk of infection. Studies on the efficacy of different types of face masks in reducing the cross-infection rate via respiratory droplets have demonstrated that loosely folded face masks or bandana-style masks provide minimal ability to stop small aerosols from traveling 4-8 feet. However, both well-fitted, homemade, stitched cotton masks with multiple layers of fabric and off-the-shelf cone-style masks (such as KN95 or N95 masks) have been shown to be effective in reducing droplet dispersal to just a few inches (Verma et al., 2020). These types of masks reduced both the speed and range of the respiratory jets, although if not worn properly, leakage around the edges can reduce their efficacy.
Other types of face protection equipment can also be highly effective in reducing the exposure to infectious aerosols, particularly in the clinical setting (Lindsley et al., 2014). Studies on influenza have demonstrated that a face shield can reduce the inhalation exposure risk by 96% during the period immediately after a cough for particles with an aerosol diameter of 8.5 microns. If worn together with an underlying face mask, face shields can reduce the contamination of the mask or respirator by 97%. Smaller particles (3.4 microns), however, are less able to be blocked by only a face shield, because particles of this size or smaller can flow around the shield and remain airborne for longer. If a face shield is combined with increasing the distance from the point of the cough’s origin to 72 inches, then exposure to those viral particles can be reduced by 92%. For those working in a clinical setting, the combination of a face shield with an underlying mask or respirator therefore provides adequate respiratory protection.
Within confined spaces, there is high variability in droplet dispersal patterns due to small changes in ambient airflow, so designing a ventilation system that minimizes the possibility of cross-infection is also critical to reducing contact with infectious particles (Verma et al., 2020). The WHO has reviewed the relationship between ventilation and airborne disease transmission in healthcare settings: increased infection rates are associated with low ventilation rates, the rate of infection decreases with distance from the source, and the airflow-induced infection rate is also dependent upon the concentration of the pathogen at the source location (WHO, 2009). Therefore, increasing ventilation and diverting airflow from high- to low-concentration areas (like outdoors) are both recommended. If not properly directed, airflow can actually increase the infection risk, as in the case of a COVID-19 outbreak in an air-conditioned restaurant in Guangzhou (Lu J et al., 2020). Thus, creating a proper direction of airflow is critical for designing the optimal ventilation system to reduce infection in a confined space.
Surface Contact Transmission of SARS-CoV-2
The SARS-CoV-2 virus is known to stay viable on surfaces for up to 72 hours. The stability of the virus depends on the material making up the surface, and it is most stable on plastic, followed by steel, copper, and cardboard surfaces (Zhang DX, 2020). Since any surface that has been exposed to the virus within the past several hours or even days is a potential source of transmission, gloves are an important means of protecting the hands from contact exposure. Hands directly exposed to contaminated surfaces that subsequently touch the nostrils, eyes, or mouth could transmit the virus. Furthermore, studies examining the local environmental spread of norovirus showed that contaminated hands could transfer the virus to up to seven clean surfaces, including commonly utilized objects such as door handles, telephone receivers, and water taps (Jones, 2020). Wearing single or double gloves in contaminated environments and promptly disposing of them after exposure are, therefore, necessary protective measures to prevent the transmission and spread of SARS-CoV-2 (Krajewska et al., 2020). The CDC recommends nitrile, polychloroprene, or rubber gloves with a minimum length of 220 mm. Hand hygiene involving the application of 60-95% alcohol hand sanitizer, or washing the hands with soap and water for at least 20 seconds, is recommended after removal of gloves (CDC, 2020). For high-risk surgeries such as orthopedic, traumatic, and emergency procedures, double gloves are highly recommended as they reduce the risk of exposure to blood and body fluids when compared to single gloves (glove perforations can occur in 5-18% of procedures) (Hirschmann et al., 2020).
Body Protection from SARS-CoV-2
Upon coughing or sneezing, an individual infected with SARS-CoV-2 will release into the air droplets 20 micrometers in size or larger, and these act as a primary source of transmission. Aerosolized SARS-CoV-2 particles smaller than 5 micrometers remain viable for 3 hours or more. Gowns, in addition to the use of protective equipment for the face and hands, are necessary to prevent these droplets and particles from contacting the body (Krajewska et al., 2020). Disposable, fluid-resistant, and impermeable gowns are recommended by the CDC. The gowns must wrap around the entire body and also cover the back, such that the back area is still protected if the wearer is in a seated or squatting position. Surgical gowns of ANSI PB70 levels 3 or 4 are needed for environments with medium to high risk of contamination (https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html; Hirschmann et al., 2020). These include situations involving exposure to bodily fluids, long-term engagement with infected patients, or surgical or invasive procedures. Surgical procedures should ideally involve surgical coveralls, which provide 360-degree body protection, while gowns have more openings and are better suited to lower-exposure activities such as patient care settings.
In addition to face, body, and hand protection, shoe coverings are also an important component of total body protection. A study examining surface contamination in hospital rooms of COVID-19 patients found that the floor had the highest likelihood of contamination (Chia et al., 2020). The shoes are a potential source of transmission to the wearer, particularly during their removal following procedures with exposure to the virus. Another study found that up to half of ICU medical staff shoe soles were positive for the virus. This study noted that while the ICU floor was generally highly positive, the hospital pharmacy floor was also positive, which suggests that shoes play a role in tracking the virus throughout large spaces (Guo et al., 2020). The continued viability of the virus after transport by shoes and the likelihood of its transmission from the floor warrants further study. It is recommended that shoe covers or booties are used and disposed of following activities in contaminated spaces, and that shoes are also disinfected to help prevent potential tracking of the virus across environments.
It is important to note that protective equipment such as disposable gowns, gloves, and shoe covers are themselves also sources of potential transmission, not only to the wearer but also to other healthcare workers and patients. Viral particles and droplets that have attached to PPE worn during procedures or healthcare activities remain detectable on the equipment surface for up to 24 hours (Hebden, 2020). The outer layer of masks have been found to remain infectious for longer than 7 days after use (Jones, 2020). Long-term use or re-use of contaminated PPE could, therefore, transmit the virus to others upon contact. Furthermore, there is risk of transmission during and after removal if the body contacts the contaminated equipment surface, further highlighting the risk of PPE re-use. This supports the practice of careful removal and prompt disposal of applicable PPE, followed by sufficient hand and body hygiene, after exposure to the virus.










