Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
Products
Antibodies
ELISA and Assay Kits
Research Areas
Infectious Disease
Resources
Purchasing
Reference Material
Contact Us
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
| Catalog Number | Size | Price |
|---|---|---|
| LS-F2223-1 | 1 Plate | $534 |



1 of 2
2 of 2
Human PTH / Parathyroid Hormone (Cell-Based ELISA) ELISA Kit - LS-F2223
Human PTH / Parathyroid Hormone (Cell-Based ELISA) ELISA Kit - LS-F2223
Description:
LS-F2223 is a 96-well enzyme-linked immunosorbent assay (ELISA) for the Qualitative detection of Human PTH / Parathyroid Hormone in samples of Adherent Cell Cultures. It is based upon a Cell-Based assay principle.
Toll Free North America
 (800) 227-6666
(800) 227-6666
For Research Use Only
Specifications
Publications
Reviews
Images
Popular PTH / Parathyroid Hormone Elisa Kits
Request SDS/MSDS
Overview
Description:
LS-F2223 is a 96-well enzyme-linked immunosorbent assay (ELISA) for the Qualitative detection of Human PTH / Parathyroid Hormone in samples of Adherent Cell Cultures. It is based upon a Cell-Based assay principle.
Specifications
Type
Cell-Based ELISA (enzyme-linked immunosorbent assay) kit
Target
PTH / Parathyroid Hormone
Synonyms
PTH | Parathyroid hormone | Parathyroid hormone 1 | Parathyrin | Parathormone | PTH1
Reactivity
Human
Kit Components
- User Manual
- 96-well Cell Culture Microplate
- Blocking Buffer
- Detection Antibodies
- HRP-Conjugate
- Substrate
- Stop Solution
- Crystal Violet Solution
- SDS Solution
- Adhesive Plate Sealers
Manual
Format
96-Well Microplate
Intended Sample Type
Adherent Cell Cultures
Detection
Colorimetric - 450nm (TMB)
Measurement
Qualitative
Storage
Short term: 4°C; Long term: see manual.
Quality Assurance
Due to their limited shelf life, LSBio ELISA kits are not typically stocked as finished goods. Upon receipt of an order each kit is assembled and tested to ensure that it meets specifications before shipping. Minor changes may occur to the Range, Sensitivity, and Precision. In the event of a significant change the order would be confirmed with the customer before shipping ELISA kit lot numbers reflect the date of final assembly and testing for each specific kit rather than a bulk manufactured lot. All kits are tested to confirm that they fall within their defined Inter- and Intra- assay coefficient of variation.
Restrictions
For research use only. Intended for use by laboratory professionals.
Guarantee
This ELISA Kit carries the LSBio 100% Guarantee
About PTH / Parathyroid Hormone
Publications (0)
Customer Reviews (0)
Images
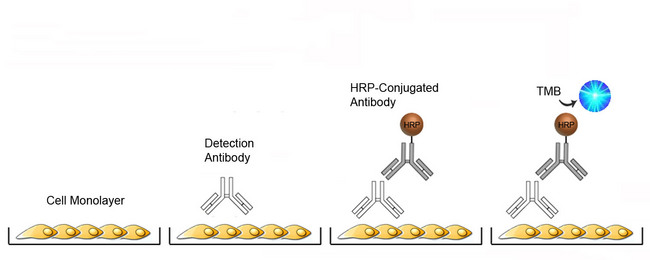
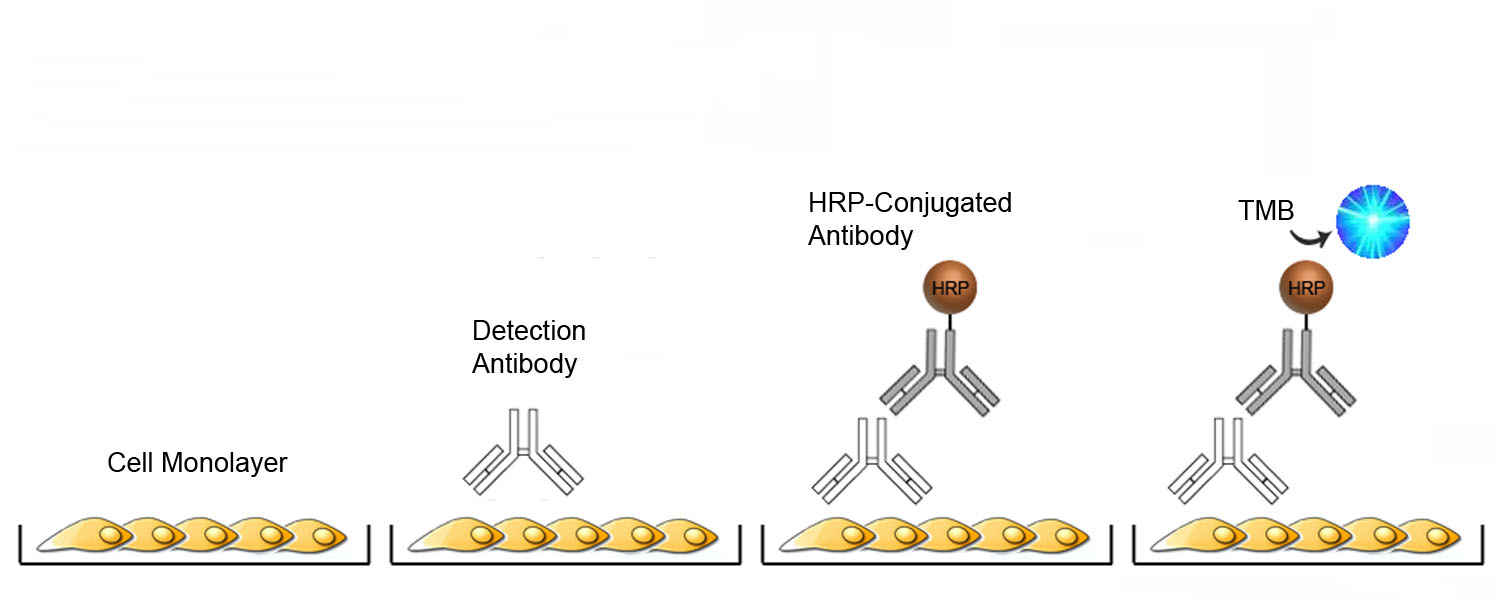
Cell-Based ELISA Platform Overview


Cell-Based ELISA Platform Overview


Cell-Based ELISA Platform Overview


Cell-Based ELISA Platform Overview
Popular PTH / Parathyroid Hormone Elisa Kits
Reactivity:
Mouse
Range:
9.88-800 pg/ml
Reactivity:
Human
Range:
1-1000 pg/ml
Reactivity:
Mouse
Range:
15.63-1000 pg/ml
Reactivity:
Rat
Range:
23.4-1500 pg/ml
Reactivity:
Rat
Range:
9.88-800 pg/ml
Reactivity:
Human
Range:
12.35-1000 pg/ml
Request SDS/MSDS
To request an SDS/MSDS form for this product, please contact our Technical Support department at:
Technical.Support@LSBio.com
Requested From: United States
Date Requested: 11/27/2024
Date Requested: 11/27/2024