Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
Products
Antibodies
ELISA and Assay Kits
Research Areas
Infectious Disease
Resources
Purchasing
Reference Material
Contact Us
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
| Catalog Number | Size | Price |
|---|---|---|
| LS-C56206-1 | 1 ml (1 mg/ml) | $444 |
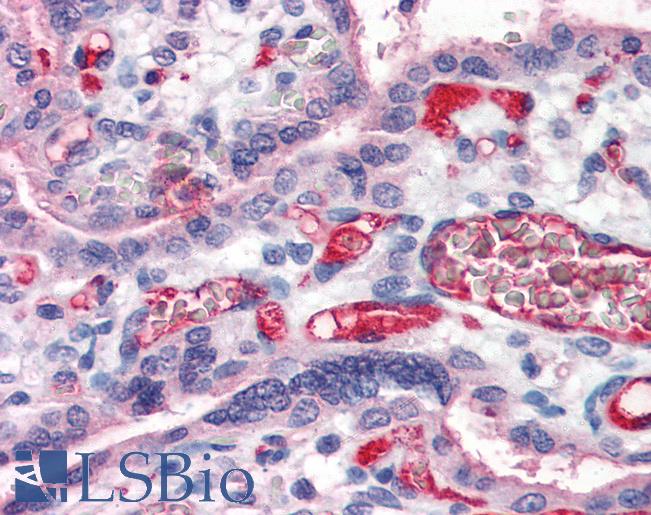
Polyclonal Sheep anti‑Human Fibrinogen Antibody (FITC, IF) LS‑C56206
Polyclonal Sheep anti‑Human Fibrinogen Antibody (FITC, IF) LS‑C56206
Antibody:
Fibrinogen Sheep anti-Human Polyclonal (FITC) Antibody
Application:
IF
Reactivity:
Human
Format:
FITC, Unmodified
Toll Free North America
 (800) 227-6666
(800) 227-6666
For Research Use Only
Overview
Antibody:
Fibrinogen Sheep anti-Human Polyclonal (FITC) Antibody
Application:
IF
Reactivity:
Human
Format:
FITC, Unmodified
Specifications
Description
Fibrinogen antibody LS-C56206 is an FITC-conjugated sheep polyclonal antibody to human Fibrinogen. Validated for IF.
Host
Sheep
Reactivity
Human
(tested or 100% immunogen sequence identity)
Clonality
IgG
Polyclonal
Conjugations
FITC
Purification
Affinity purified
Modifications
Unmodified
Immunogen
Native
Specificity
Recognizes human fibrinogen, a complex ~340kD hetero-hexameric (di-trimeric) glycoprotein consisting of 3 pairs of alpha, beta and gamma chains linked by a series of 29 disulphide bonds. The six chains are arranged in such a way that all the N-Terminal ends adjoin to form a central with two trimeric coiled coil structures connecting to outer D domains. Fibrinogen plays an important role in the coagulation process with the D and E domains interacting via the C-Terminal ends of the alpha chains during fibrin clot cross-linking. Shows minimal cross-reactivity with related serum proteins. Fibrinogen has been identified as a ferritin binding protein in the horse. Has been successfully as a capture reagent for ferritin - anti ferritin IgG complexes in horse plasma to evaluate the antibody response to ferritin by ELISA.
Applications
- Immunofluorescence (1:10 - 1:100)
Usage
The applications listed have been tested for the unconjugated form of this product. Other forms have not been tested.
Presentation
PBS, 0.09% Sodium Azide
Storage
Store at 4°C or at -20°C. Store undiluted. Avoid freeze-thaw cycles. Microcentrifugation recommended if solution contains precipitate.
Restrictions
For research use only. Intended for use by laboratory professionals.
Publications (0)
Customer Reviews (0)
Featured Products
Species:
Human
Applications:
IHC, IHC - Paraffin, Western blot, ELISA
Species:
Mouse, Rat
Applications:
IHC, IHC - Paraffin, IHC - Frozen, Western blot, ELISA
Request SDS/MSDS
To request an SDS/MSDS form for this product, please contact our Technical Support department at:
Technical.Support@LSBio.com
Requested From: United States
Date Requested: 1/29/2025
Date Requested: 1/29/2025